Ch 3 Lecture 2 Bonding Considerations - PowerPoint PPT Presentation
1 / 15
Title:
Ch 3 Lecture 2 Bonding Considerations
Description:
SN = 5, so structure is base on trigonal bipyramid. Three possible structures ... trigonal pyramid. see-saw. T-shaped. linear. square pyramid. square planar ... – PowerPoint PPT presentation
Number of Views:86
Avg rating:3.0/5.0
Title: Ch 3 Lecture 2 Bonding Considerations
1
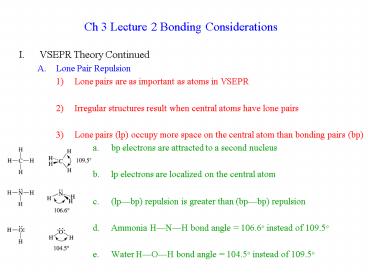
Ch 3 Lecture 2 Bonding Considerations
- VSEPR Theory Continued
- Lone Pair Repulsion
- Lone pairs are as important as atoms in VSEPR
- Irregular structures result when central atoms
have lone pairs - Lone pairs (lp) occupy more space on the central
atom than bonding pairs (bp) - a. bp electrons are attracted to a second nucleus
- lp electrons are localized on the central atom
- (lpbp) repulsion is greater than (bpbp)
repulsion - Ammonia HNH bond angle 106.6o instead of
109.5o - Water HOH bond angle 104.5o instead of 109.5o
2
- Subtle Geometric Influences
- Size of the central atom
- Larger central atom leads to longer bonds and
less bpbp repulsion - Smaller bond angles result
- Example H2O, H2S, H2Se, H2Te
- Electronegativity of outer atoms
- More electronegative outer atom pulls bp towards
it - Reduces bpbp repulsion and makes smaller bond
angles - Example PF3, PCl3, PBr3, PI3
- Size of outer atoms
- Very small outer atoms (H) may reverse expected
trends - Example SH2, SF2, SCl2
- Exact predictions are rarely needed, qualitative
predictions are often enough. - Table 3-4 gives geometric data
3
(No Transcript)
4
- Choose the structure that allows maximum lone
pair separation - Example ClF3
- SN 5, so structure is base on trigonal
bipyramid - Three possible structures
- lplp most important eliminates B as a
possibility - 90o interactions are very unfavorable
- Angles gt 90o give about the same interaction
- Structure A has 6 90o lpbp interactions
- Structure C has 4 900 lpbp interactions
5
- 2. Always place lone pairs in equatorial
positions in SN 5 and SN 7
linear
bent
trigonal pyramid
bent
T-shaped
linear
see-saw
square pyramid
square planar
6
- Remember lone pairs count in SN, but are left
out in structure name - Examples SbF4, SF5, SeF3
- Exercise 3-2
- Multiple Bonds
- Double and Triple bonds have greater repulsion
than - single bonds due to the large p-orbitals involved
- Multiple bonds are placed to minimize
interactions, similar to lp - Multiple bonds are placed equatorially in SN5,
SN7 - lp gt mp gt bp
- Examples HCP, IOF4-, SeOCl2
- Exercise 3-3
7
(No Transcript)
8
- II. Electronegativity
- Definitions
- Electronegativity power of an atom in a molecule
to attract electrons - Polar Bond between pure covalent (sharing e-)
and ionic (transfer of e-) - Homonuclear Diatomics completely nonpolar HH
- Heteronuclear Diatomics polar HCl
- Paulings Electronegativity Scale
- Definitions
- D Bond Dissociation Energy (AA ? A A)
- D Ionic Resonance Energy (kJ)
- cA Electronegativity of A
- Equations
9
- F set at c 4.0 and other atoms determined from
it - Example Find D of HCl using table 3-1 (cH
2.20, cCl 3.16) - Exercise 3-4 Find D of HO. (HH 432kJ/mol,
OO 213 kJ/mol)
10
- Large c differences give ionic bonds small c
differences give polar covalent bonds - Actual c values come from averaging several
compounds of the atom to remove experimental
error - Actual c values in a given molecule may vary from
the average - Noble gases have high x values because of high
effective nuclear charges - Small size
- Large nucleus
11
- Polar Bonds
- Any bond between atoms of different
electronegativities is polar - Electrons concentrate on one side of the bond
- One end of the molecule is () and one end is (-)
- Calculating Dipole Moments
- Dielectric Constant Capacitance of Full Cell /
Capacitance of Vacuum - The orientation of polar molecules cancels out
capacitance and this difference gives the
dielectric constant - The temperature dependence of this value lets us
calculate dipole moment (m) - m Qr units Cm or D(debye)
- Q difference in charge
- r distance of separation
- 1 D 3.38 x 10-30 Cm
- Complex molecules vector addition of all m
- Not very accurate (table 3-5) because of
variation lengths and angles - Lone pairs have a large influence on dipole
moment as well
12
- Examples
- Large dipoles result from lone pair reinforcement
of bond dipoles - Small dipoles result from lone pair cancellation
of bond dipoles - Symmetric molecules have have no net dipole
13
- Hydrogen Bonding
- Hydrogens bonded to an electronegative atom (O,
N, F, Cl, Br,) can form a weak bond to the lone
pair on another electronegative atom - Water boils about 200 oC higher than expected
without this interaction - Ammonia does not H-Bond with itself well, instead
forming a NN dimer
14
- Unusual properties of water due to H-Bonding
- Freezing point gtgt similar compounds
- Decreases in density on freezing
- H-Bonding network gives an open structure
- Ice floats (think biology and geology)
15
- Other H-Bonding Phenomena include Protein and
Nucleic Acid Folding