Scenario 4 - PowerPoint PPT Presentation
1 / 40
Title: Scenario 4
1
Scenario 4
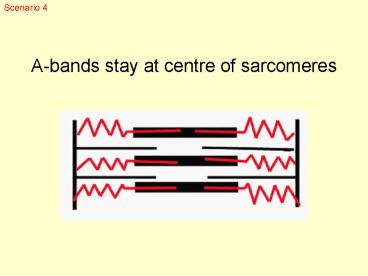
A-bands stay at centre of sarcomeres
2
Scenario 4
3
Scenario 4
Hierarchical organization of striated
muscle Muscle is a tissue, i.e. population of
cells The cells are known as muscle fibres
(Aquire highly elongated shape by end-to-end
fusion of myoblasts) The cells contain
myofibrils, which are organelles, (just as are
mitochondria, except no membranes)
4
Scenario 4
Hierarchical organization of sarcomeric muscle
5
Scenario 4
Myogenesis
6
Scenario 4
Myofibril
7
Scenario 4
Distinguish the structural levels of sarcomeric
muscle "Fibre/Fibril/Filament"
8
Scenario 4
Hierarchical organization of striated
muscle Sarcomeres are repeated structural unit
of myofibrils and contain several types of
filaments
9
Scenario 4
I - Isotropic H - Hell pale A -
Anisotropic
10
Scenario 4
Draw a diagram of a sarcomere, showing thick and
thin filaments Z-disc A and I bands
11
Scenario 4
Thin filaments are microfilaments, assembled
during development from G-actin (Scenario 3), but
no longer dynamic, and of accurately fixed
length Also contain tropomyosin and troponin,
which together confer calcium-dependence on
contraction Troponin C is muscle isoform of
calmodulin
12
Scenario 4
13
Scenario 4
Thick filaments Bipolar filaments assembled by
precise bundling of tails of the motor protein
myosin II Two stages of interaction 1 Two
myosin heavy chains (MHC) join by coiled - coil
of ?-helices (heptad repeat) Hydrophobic
interaction. Two chains form one myosin
molecule.
14
Scenario 4
15
Scenario 4
- Thick filaments
- Two-stages of interaction
- Molecules associate, largely by electrostatic
interaction (attraction of opposite charges). - Myosin molecules are soluble in high salt
- 0.6M KCl, on dilution to 0.1M,
- filaments self- assemble
16
Scenario 4
R, K or H
D or E
28-residue periodicity of charged residues in
myosin II tail
17
Scenario 4
18
Scenario 4
Scenari 5
19
Scenario 4
Describe in terms of physico-chemical
interactions the assembly of myosin molecules
(coiled-coil tails) thick filaments (alternating
bands of opposite charge)
20
Scenario 4
21
Scenario 4
Myosin superfamily As kinesin, the myosin motor
domain, occurs in a large number of different
molecules, with varied functions. At least 17
families. Most are plus end- (barbed end)
directed motors. Many do not form filaments, and
are involved in, e.g. vesicle transport on actin.
22
Scenario 4
23
Scenario 4
24
Scenario 4
25
Scenario 4
Myosin about mechanism Historically, studied as
muscle actomyosin (gel contracts on adding ATP-Mg
2 Ca 2) Motor assays, cf kinesin
26
Scenario 4
27
Scenario 4
28
Scenario 4
29
Scenario 4
Force exerted proportional to number of heads on
actin
30
Scenario 4
- Muscle length/tension relationship
- Force proportional to number of myosin motor
domains reaching actin - In normal function (A-B on diagram) is
proportional to extent of overlap of thick and
thin filaments - If an A-band moves slightly off centre, it
experiences greater force in the direction of the
error. - Therefore need a centring mechanism - elastic
protein titin
31
Scenario 4
Explain in terms of cross-bridge number, why
A-band centration requires stabilization
32
Scenario 4
33
Scenario 4
34
Scenario 4
Describe evidence (from trypsin-treated,
"skinned" muscle) that a protein stabilizes
centration
35
Scenario 4
- Titin
- Longest single polypeptide known.
- gt 3 Megadaltons 3 x 106
- In sarcomeric muscles. i.e worm, fly, man, not
yeast! - One end links to Z-disc, the other to centre of A
band - gt300 domains in tandem along length.
- Domains immunoglobulin-like (IgG) or Fibronectin
type III
36
Scenario 4
37
Scenario 4
IgG domains of I band denature reversibly under
tension
38
Scenario 4
Describe the reversible domain-unfolding model
of the elastic role of titin
39
Scenario 4 END
A-bands stay at centre of sarcomeres
40
(No Transcript)