Chapter Three - PowerPoint PPT Presentation
1 / 51
Title:
Chapter Three
Description:
And Reaction Stoichiometry ... Solve the same as a normal stoichiometry problem except... Now solve the stoichiometry problem: How many grams of Mg3N2 can ... – PowerPoint PPT presentation
Number of Views:59
Avg rating:3.0/5.0
Title: Chapter Three
1
Chapter Three
- StoichiometryChemical Calculations
2
Molecular Masses AndFormula Masses
- Molecular Mass
- The sum of the masses of the atoms represented in
a molecular formula. - Specifically for molecules.
- Formula Mass
- The sum of the masses of the atoms or ions
present in a formula unit. - Used for ionic compounds (dont exist as
molecules) - What are the units of molecular/formula mass?
3
Molecular Masses AndFormula Masses
- Carbon dioxide, molecular mass 44.0 u
- Oxygen gas, molecular mass 32.0 u
- Bromine trichloride, molecular mass 186.3 u
- Magnesium oxide, formula mass 40.3 u
- Calcium carbonate, formula mass 100.1 u
- Copper(II) sulfate pentahydrate
- formula mass 249.7 u
4
The Mole Avogadros Number
- A mole (mol) is an amount of substance that
contains as many elementary entities as there are
atoms in exactly 12 g of the carbon-12 isotope. - Atoms are small, so this is a BIIIIG number
- Avogadros Constant (NA)
- NA 6.02214199 x 1023 mol-1
- 1 mol 6.02214199 x 1023 elementary entities.
- Warning! Mole is NOT abbreviated as either M or
m.
5
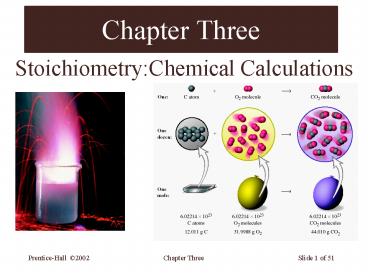
The Mole and Formation of Carbon Dioxide
6
Molar Mass
- The mass of one mole of that substance.
- The molar mass is numerically equal to the atomic
mass, molecular mass, or formula mass. - The units of molar mass are grams/mole (g/mol).
- Examples
- 1 mol Na 22.99 g/mol
- 1 mol CO2 44.01g/mol
7
Converting mass ?? moles
Use molar mass as a conversion factor!
Moles of A
Conversion factor from
molar mass of A
Mass of A
Note preliminary and followup calculations may
be needed
8
Converting mass ?? moles
9
Mass Percent CompositionFrom Chemical Formulas
- The mass percent composition of a compound refers
to the proportion of the constituent elements
expressed as the number of grams of each element
per 100 grams of the compound. - Mass fraction mass of element / mass of
compound - Mass percent mass fraction x 100
10
Mass Percent CompositionFrom Chemical Formulas
SCHEMATIC REPRESENTATION OF
Percentage of carbon in butane, C4H10
Percentage of hydrogen in butane, C4H10
11
Determining Empirical Formulas
- Reverse of finding percentage composition.
- Determine moles of each element (from percentage
or mass). - Find the smallest whole-number ratio of moles by
dividing each number of moles by the smallest
number.
12
Determining Empirical Formulas
13
Determining Empirical Formulas (contd)
Caution dont round off these values
14
Relating Molecular FormulasTo Empirical Formulas
- Molecular formulas are simple integer multiples
of the empirical formulas. - That is, an empirical formula of CH2 means that
the molecular formula is CH2 or C2H4 or C3H6 or
C4H8 etc. - Find the molecular formula by
- molecular formula mass/empirical formula mass
number of empirical formula units in molecular
formula - Example A compound has an empirical formula of
CH and a molecular mass of about 77 u. What is
the molecular formula? - 77 u / 13 u 5.92 or 6 empirical formula units
C6H6
15
Elemental Analysis
- A method of determining empirical formula in the
laboratory. - Used primarily for organic compounds (contain
carbon, hydrogen, oxygen). - The products of combustion are weighed, and the
amount of each element is determined. - Question When an organic compound is burned,
exactly what are the products of combustion?
16
Elemental Analysis
Oxygen gas enters the combustion apparatus and
streams over the sample under analysis in a
high-temperature furnace. The absorbers collect
the water vapor and carbon dioxide that are
produced in the combustion.
How do we get mass of oxygen?
(Gives mass of hydrogen)
(Gives mass of carbon)
17
Writing Chemical Equations
- A chemical equation is a shorthand description of
a chemical reaction, using symbols and formulas
to represent the elements and compounds involved. - Starting substances are called reactants.
- The substances formed from the reaction are
called products. - A plus sign () is used between each reactant and
between each product. - An arrow (?) points from the reactants to the
products.
18
Balancing Equations Illustrated
Equations may be balanced only by changing the
coefficients.
19
Chemical Equation Notations
- Extra notation sometimes added to each substance
- (g) gas
- (l) liquid
- (s) solid
- (aq) aqueous (water) solution
- Extra notation added above the arrow
- ? heat
- Sometimes the actual temperature is used instead
20
Balancing Chemical Equations
- Stoichiometric coefficients are numbers placed in
front of formulas in a chemical equation to
balance the equation. - The coefficients indicate the combining ratios of
the reactants and the ratios in which products
are formed. - For each element, the same number of atoms must
appear on each side of the equation.
21
Guidelines For BalancingChemical Equations
- If an element is present in just one compound on
each side of the equation, try balancing that
element first. - Balance any reactants or products that exist as
the free element last. - In some reactions, certain groupings of atoms
(such as in polyatomic ions) remain unchanged. In
such cases, balance these groupings as a unit. - At times, an equation can be balanced most
readily by first using a fractional
coefficient(s) and then clearing the fractions by
multiplying all coefficients by a common
multiplier.
22
Stoichiometric EquivalenceAnd Reaction
Stoichiometry
- Stoichiometric factors, or mole ratios, are
conversion factors formed from the stoichiometric
coefficients in a chemical equation. - In the equation CO(g) 2H2(g) ? CH3OH(l)
- 1 mol CO is chemically equivalent to 2 mol H2
- 1 mol CO is chemically equivalent to 1 mol CH3OH
- 2 mol H2 is chemically equivalent to 1 mol CH3OH
- These conversion factors are commonly used in
problem-solving.
23
Concept of Stoichiometric Equivalence
One car may be equivalent to either 25 feet or 10
feet, depending on the equation (method of
parking).
One mole of CO may be chemically equivalent to
one mole of CH3OH or to one mole of CO2 it
depends on the equation.
24
Outline Of Simple Reaction Stoichiometry
Moles of A
Conversion factor from
balanced equation
Moles of B
Note preliminary and followup calculations may
be needed
25
Simple Reaction Stoichiometry
26
Outline of stoichiometry involving mass
Mass of A
Conversion factor from
molar mass of A
Moles of A
Conversion factor from
balanced equation
Moles of B
Conversion factor from
molar mass of B
Mass of B
Note preliminary and followup calculations may
be needed
27
Stoichiometry involving mass
28
Stoichiometry involving mass
29
Limiting Reactants
- Many reactions are carried out with a limited
amount of one reactant and a plentiful amount of
the other(s). - The reactant that is completely consumed in the
reaction limits the amounts of products and is
called the limiting reactant, or limiting
reagent. - Keep in mind that the limiting reactant is not
necessarily the one present in smallest amount.
30
Limiting Reactant Analogy
Limiting reactant determines
the amount of product
31
Recognizing and Solving Limiting Reactant Problems
- Recognize by the fact that amounts of two (or
more) reactants are given. - Solve the same as a normal stoichiometry problem
except - preliminary calculations are needed to
determine which of the two reactants is limiting
(which reactant should be used to do the
stoichiometry calculation).
32
Recognizing and Solving Limiting Reactant
Problems (contd)
- Select one reactant.
- Determine the amount (moles, grams, mL, etc.) of
the other reactant is needed to consume the first
reactant. - Compare the amount needed to the amount you
have, and ask - IS THERE ENOUGH?
- The reactant thats not enough is consumed
completely it is the limiting reactant. - Now do the stoichiometry based on the limiting
reactant.
33
Solving Limiting Reactant Problems
Question What mass of nitrogen will react with
35.00 g of Mg?
34
Solving Limiting Reactant Problems (contd)
Question What mass of nitrogen will react with
35.00 g of Mg?
Answer 1 mol Mg 1 mol N2 28.0 g
N2 35.00 g Mg x x
x 13.4 g N2 24.3 g Mg 3 mol Mg
1 mol N2
We need 13.4 g N2. We have 15.00 g N2. Clearly,
there is more N2 than is needed. Therefore Mg is
the limiting reactant.
35
Solving Limiting Reactant Problems (contd)
Now solve the stoichiometry problem How many
grams of Mg3N2 can be made from 35.00 g Mg? 1
mol Mg 1 mol Mg3N2 100.9 g Mg3N2 35.00 g Mg
x x x
24.3 g Mg 3 mol Mg 1 mol Mg3N2
48.4 g Mg3N2 The preliminary limiting-reactant
calculation was needed to find out which reactant
to use in this calculation the Mg.
36
Yields Of Chemical Reactions
- Theoretical yield of a chemical reaction the
calculated quantity of product in the reaction. - Actual yield the amount you actually get when
you carry out the reaction. - Actual yield will always be less than the
theoretical yield, for many reasons (can you name
some?) - Percent yield
37
Solutions AndSolution Stoichiometry
- Solute the substance being dissolved
- Solvent the substance doing the dissolving
- The concentration of a solution refers to the
quantity of a solute in a given quantity of
solvent. - A concentrated solution contains a relatively
large amount of solute as compared to the
solvent. - A dilute solution contains a relatively small
concentration of solute as compared to the
solvent.
38
Molar Concentration
- Molarity (M), or molar concentration, is the
amount of solute, in moles, per liter of
solution. - Keep in mind that molarity signifies moles of
solute per liter of solution, not liters of
solvent.
39
Molar Concentration
40
Dilution Of Solutions
- Dilution is the process of preparing a more
dilute solution by adding solvent to a more
concentrated one. - Addition of solvent does not change the amount of
solute in a solution but does change the solution
concentration.
41
Visualizing The Dilution Of A Solution
Same amount of solute in a larger volume of
solution
means lower concentration.
42
Dilution of Copper (II) sulfate
Notice that the solute particles are ions
copper(II) and sulfate
Same amount of solute in a larger volume of
solution.
43
Dilution calculations
Moles of solute does not change on dilution, so
44
Dilution calculations (contd)
45
Solutions In Chemical Reactions
- Molarity provides an additional tool in
stoichiometric calculations based on chemical
equations. - Molarity provides conversion factors for
converting between moles of solute and liters of
solution.
46
Mass of A
Outline of Stoichiometry Including Solutions
Conversion factor from molar mass of A
Mmol/L
Moles of A
V and M of solution A
Conversion factor from
balanced equation
V or M of solution B
Moles of B
Mmol/L
Conversion factor from molar mass of B
Mass of B
Note preliminary and followup calculations may
be needed
47
Example of Solution Stoichiometry
48
Example of Solution Stoichiometry (contd)
1. Volume and concentration of A
2. Conversion factor from balanced equation
3. We stop at mol of B because thats the unit
we want.
49
Example of Solution Stoichiometry (contd)
Start with mol of A
Molarity
From balanced equation
50
Summary
- Molecular and formula masses relate to the masses
of molecules and formula units. - A mole is an amount of a substance containing
Avogadros number of elementary entities. - Avogadros constant (NA) 6.022 x 1023.
- The molar mass of a substance is the mass in
grams of one mole of that substance. - Formulas and molar masses can be used to
calculate mass percent composition.
51
Summary (continued)
- A chemical equation uses symbols and formulas for
the elements and/or compounds involved in a
reaction. - Calculations involving reactions use
stoichiometric factors based on stoichiometric
coefficients in the balanced equation. - The limiting reactant determines the amounts of
products in a reaction. - The molarity of a solution is the number of moles
of a solute per liter of solution.