Nucleophilic Substitution - PowerPoint PPT Presentation
1 / 18
Title:
Nucleophilic Substitution
Description:
On going from 1 to 2 and 3 haloalkanes, the steric crowding about. the carbon increases. increase in steric hindrance for approaching nucleophile ... – PowerPoint PPT presentation
Number of Views:234
Avg rating:3.0/5.0
Title: Nucleophilic Substitution
1
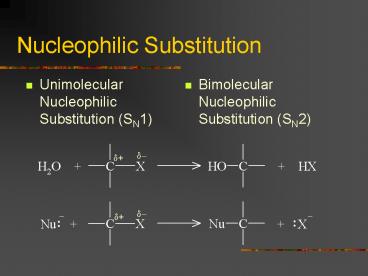
Nucleophilic Substitution
- Unimolecular Nucleophilic Substitution (SN1)
- Bimolecular Nucleophilic Substitution (SN2)
??
?
??
?
?
?
2
Nucleophilic Substitution
- The nucleophile may be a molecule or anion. It
has an unshared pair of electrons, which tends to
seek the positive centre of halogenoaklanes to
react.
??
?
?
3
Bimolecular Nucleophilic Substitution (SN2)
OH? CH3CH2Br ? CH3CH2OH Br? Rate k
CH3CH2BrOH? Mechanism
?
??
4
Bimolecular Nucleophilic Substitution (SN2)
Energy profile
Potential energy
OH? C2H5Br?
Br? C2H5OHr?
Reaction coordinate
5
Unimolecular Nucleophilic Substitution (SN1)
Nu? ?
Br? Rate k
6
Unimolecular Nucleophilic Substitution (SN1)
Mechanism Step 1 (r.d.s.)
?
?
??
7
Unimolecular Nucleophilic Substitution (SN1)
Mechanism Step 2 (fast)
?
8
Unimolecular Nucleophilic Substitution (SN1)
Energy profile
Nu?
P.E.
Br?
Nu?
Nu?
?
Br?
Br?
Reaction coordinate
9
SN1 v.s. SN2
??
?
10
SN1 v.s. SN2
Effect of concentration and reactivity of the
attacking nucleophile
- In SN1 reactions, since the nucleophile does
not take part in the - rate determining step, the rate of an SN1
reaction is unaffected - by either the concentration or the identity
of the nucleophile. - In SN2 reactions, the rate is of course
dependent on both the - concentration and the identity of the
nucleophile, which takes - part in the rate determining step.
- In general, negatively charged nucleophiles
(e.g. OH?) are - stronger than uncharged nucleophiles (e.g.
H2O), because - the former are more readily attracted to the
?? carbon in the - C?X bond.
- In some cases the order of nucleophilicity
roughly follows - the order of basicity
- RO?gtHO?gtgtROHgt H2O
11
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
, halobenzene is very unreactive towards SN
reaction.
- The C?X bond has a partial double bond character.
- X has a lone pair electrons delocalized into the
? system. - This decreases the ?? and ?? in the C?X
bond.
12
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
, (halomethyl)benzene is very reactive towards
SN1 reaction.
?
?
?
The positive charge in the intermediate can be
dispersed through the ? system. ?The
intermediate is stabilized effectively.
13
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
, 3O halogenoalkane is very reactive towards SN1
reaction.
?
14
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
15
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
On going from 1º to 2º and 3º haloalkanes, the
steric crowding about the ?? carbon increases.
? increase in steric hindrance for approaching
nucleophile ? ? great decrease in SN2 reaction
rate ?
16
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
17
SN1 v.s. SN2
Effect of structure around the carbon which is
being attacked
SN1 only
log(reaction rate)
SN2 only
bromoalkanes
18
SN1 v.s. SN2
Nature of leaving group
For both SN1 and SN2 reactions, the easier the
C?X bond to break, the faster the SN reaction. Of
the halogens, the ease of leaving is
I?gtBr?gtCl?gtF?