Precipitation in AlCu System - PowerPoint PPT Presentation
1 / 8
Title:
Precipitation in AlCu System
Description:
To use Al-Cu system to illustrate the process of precipitation which leads to ... Precipitates h' and h (MgZn2) in a 7150-T651 Al alloy - TEM micrograph. 100 nm ... – PowerPoint PPT presentation
Number of Views:576
Avg rating:3.0/5.0
Title: Precipitation in AlCu System
1
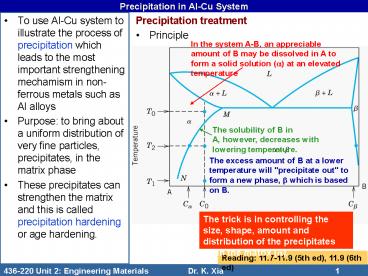
Precipitation in Al-Cu System
- To use Al-Cu system to illustrate the process of
precipitation which leads to the most important
strengthening mechamism in non-ferrous metals
such as Al alloys - Purpose to bring about a uniform distribution of
very fine particles, precipitates, in the matrix
phase - These precipitates can strengthen the matrix and
this is called precipitation hardening or age
hardening.
- Precipitation treatment
- Principle
In the system A-B, an appreciable amount of B may
be dissolved in A to form a solid solution (a) at
an elevated temperature
The solubility of B in A, however, decreases with
lowering temperature.
The excess amount of B at a lower temperature
will "precipitate out" to form a new phase, b
which is based on B.
The trick is in controlling the size, shape,
amount and distribution of the precipitates (b)
in the matrix (a).
Reading 11.7-11.9 (5th ed), 11.9 (6th ed)
2
Precipitation in Al-Cu System
- The process
1. Solution treatment heating to a T in the
single a region (e.g. T0 for alloy C0) and
holding for sufficient time to obtain a single a
phase.
2. Quenching rapid cooling to a low T (normally
RT, e.g. T1) to keep the single a phase which is
now supersaturated.
3. Ageing raising T to an elevated level (e.g.
T2) to bring about b precipitates in the matrix
of a.
When T2 gt T1 Artificial ageing When T2 T1
RT Natural ageing
3
Precipitation in Al-Cu System
a
b
a
Artificial ageing
Natural ageing
4
Precipitation in Al-Cu System
100 nm
Precipitates h' and h (MgZn2) in a 7150-T651 Al
alloy - TEM micrograph
5
Precipitation in Al-Cu System
- Al-Cu system
Al-4Cu
6
Precipitation in Al-Cu System
- Sequence of precipitation in Al-4Cu
GP zones
q"
Supersaturated a
q'
q
7
Precipitation in Al-Cu System
- Age hardening
- strength increases initially with ageing
- strength reaches a peak
- strength decreases eventually
a mixture of q" and q' at the peak
Peak ageing
Underageing
8
Precipitation in Al-Cu System
- effect of temperature
The practical ageing temperature is 190C and
ageing time 12 hours