16. Oncogenes and Cancer - PowerPoint PPT Presentation
1 / 28
Title:
16. Oncogenes and Cancer
Description:
Stages in the evolution of colon cancer. ii). Growth characteristics of cells in culture ... p53 (17p) transcription Li-Fraumeni breast, colon, syndrome & lung cancer ... – PowerPoint PPT presentation
Number of Views:611
Avg rating:3.0/5.0
Title: 16. Oncogenes and Cancer
1
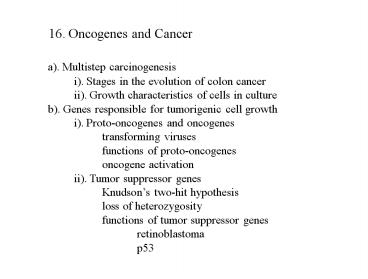
16. Oncogenes and Cancer
a). Multistep carcinogenesis i). Stages in the
evolution of colon cancer ii). Growth
characteristics of cells in culture b). Genes
responsible for tumorigenic cell growth i).
Proto-oncogenes and oncogenes transforming
viruses functions of proto-oncogenes oncogen
e activation ii). Tumor suppressor
genes Knudsons two-hit hypothesis loss of
heterozygosity functions of tumor suppressor
genes retinoblastoma p53
2
Multistep carcinogenesis
Stages in the evolution of colon cancer
Chromosome 5q gene loss or mutation
Normal colon cell
Increased cell growth
Ras gene mutation
Adenoma I
Chromosome 18 loss or mutation DCC tumor
suppressor gene
Adenoma II
Adenoma III
Carcinoma
Chromosome 17 loss or mutation p53 tumor
suppressor gene
Metastasis
Other chromosome losses
3
- Growth characteristics of cells in culture
- (parallels what happens in vivo)
- Normal primary cells survive only a relatively
short - time in culture (days to weeks)
- Immortalized (established) cells immortalized,
but not transformed - (they retain many of their in vivo
characteristics) - anchorage dependence (prefer to adhere to
culture plate) - serum dependence (require growth factors)
- contact inhibited (stop growing when cells are
confluent) - Transformed cells will form tumors
(tumorigenic) when injected - into a host, but will not necessarily kill the
host - less dependent on substratum
- less dependent on growth factors
- not contact inhibited
- Metastatic cells fully transformed cells that
have the ability to - migrate and invade tissues will establish new
colonies - and kill the host
At least 2-3 steps (which are caused by mutations)
4
What are the genes responsible for tumorigenic
cell growth?
Normal
Proto-oncogenes
Cell growth and proliferation
Tumor suppressor genes
Cancer
Mutated or activated oncogenes
Malignant transformation
Loss or mutation of Tumor suppressor genes
5
- Oncogenes are usually dominant (gain of function)
- cellular proto-oncogenes that have been mutated
(and activated) - cellular proto-oncogenes that have been captured
by retroviruses - and have been mutated in the process (and
activated) - virus-specific genes that behave like cellular
proto-oncogenes - that have been mutated to oncogenes (i.e.,
activated) - Tumor suppressor genes are usually recessive
(loss of function) - loss of a cellular gene or chromosome region by
deletion - loss of gene function by an inactivating point
mutation
6
Transforming viruses Viral class Viral
genome Oncogenes adenovirus ds DNA
E1A E1B papovavirus ds DNA T antigens
SV40 (monkey) Polyoma (human) retrovirus
ss RNA mutated cellular proto-oncogenes
7
Activities of viral oncogenes
Oncogene activity Virus
Immortalizing Transforming
adenovirus (human) E1A
E1B papovavirus SV40 (monkey) large
T antigen small T antigen Polyoma (human)
large T antigen middle T antigen retrovirus
Avian myelocytomatosis myc Harvey
murine sarcoma ras
8
- Retrovirus oncogenes derived from normal
cellular genes - Retrovirus Viral oncogene
Cellular proto-oncogene - Rous sarcoma virus v-src c-src
(src) - Simian sarcoma v-sis c-sis (sis)
- Harvey murine sarcoma v-H-ras
c-H-ras (H-ras) - Kirsten murine sarcoma v-K-ras
c-K-ras (K-ras) - FBJ murine osteosarcoma v-fos c-fos
(fos) - Avian myelocytomatosis v-myc c-myc
(myc) - Abelson leukemia virus v-abl c-abl
(abl) - Avian erythroblastosis v-erbB
c-erbB (erbB) - viral oncogenes are 80-99 homologous to
cellular proto-oncogenes - viral oncogenes in general are copies of
cellular mRNA and lack introns
9
Gene organization of a retrovirus
5
3
Gene organization of a transforming retrovirus
gag pol env onc
5
3
gag group specific antigen pol reverse
transcriptase env envelope onc oncogene
Gene organization of a cellular proto-oncogene
mRNA
5
3
10
Functions of cellular proto-oncogenes
1. Secreted Growth Factors
2. Growth Factor Receptors
4. Nuclear Proteins Transcription Factors
3. Cytoplasmic Signal Transduction Proteins
Cell Growth Genes
11
Functions of selected
proto-oncogenes Proto-oncogene
Biochemical property 1. Secreted
growth factors c-sis Platelet derived
growth factor 2. Growth factor
receptors c-erbB Epidermal growth factor
receptor 3. Signal transduction
proteins c-abl Protein kinase c-src Protei
n kinase H-ras Small G-protein K-ras Small
G-protein 4. Nuclear proteins c-myc Transcri
ption factor c-fos Transcription factor
12
- Oncogenes in human tumors
- Mechanisms of activation of proto-oncogenes
- point mutations
- chromosomal rearrangements or translocations
- gene amplifications
13
- Identification of oncogene mutations in human
tumors - most human tumors contain mutated or activated
proto-oncogenes - demonstrated by isolating the mutated genes from
human tumors
Isolate DNA
Transfer fragments of DNA into normal cells
Tumor cells
Isolate transformed clones of cells (by virtue of
their growth advantage)
Isolate new DNA gained by transformed cells
10-20 of spontaneous human tumors have DNA that
will transform cells in culture most are due to
ras gene mutations
14
Ras family proteins
- the c-ras family contains three genes H-ras,
K-ras, and N-ras - the Ras proteins encoded by these genes are
small G-proteins - the proteins transmit growth signals from cell
surface receptors - the Ras proteins are activated by binding GTP
- the proteins are inactivated by GTP to GDP
hydrolysis - mutations in the c-ras genes inactivate the Ras
GTPase - mutated Ras proteins are constitutively active
- constitutively active Ras proteins result in
uncontrolled cell growth
15
Amino acid substitutions in Ras family proteins
amino acid position Ras gene 12
59 61 Tumor c-ras (H, K,
N) Gly Ala Gln normal cells H-ras Gly Ala Leu
lung carcinoma Val Ala Gln bladder
carcinoma K-ras Cys Ala Gln lung
carcinoma Arg Ala Gln lung carcinoma Val Al
a Gln colon carcinoma N-ras Gly Ala Lys neurob
lastoma Gly Ala Arg lung carcinoma Mur
ine sarcoma virus H-ras Arg Thr Gln Harvey
strain K-ras Ser Thr Gln Kirsten strain
16
Chromosomal rearrangements or translocations Neo
plasm Translocation Proto-oncogene Burkitt
lymphoma t(814) 80 of cases
c-myc1 t(822) 15 of cases t(28) 5
of cases Chronic myelogenous t(922) 90-95 of
cases bcr-abl2 leukemia Acute
lymphocytic t(922) 10-15 of cases
bcr-abl2 leukemia 1c-myc is translocated
to the IgG locus, which results in its activated
expression 2bcr-abl fusion protein is produced,
which results in a constitutively active abl
kinase
17
c-myc is translocated to the IgG locus, which
results in its activated expression
c-myc
IgG
IgG enhancer
c-myc is activated by the IgG enhancer
in lymphocytes
bcr-abl fusion protein is produced, which results
in a constitutively active abl kinase
bcr
bcr-abl
abl
18
Gene amplification Oncogene Amplification
Source of tumor c-myc
20-fold leukemia and lung carcinoma N-myc
5-1,000-fold neuroblastoma retinoblastoma
L-myc 10-20-fold small-cell lung cancer
c-abl 5-fold chronic myoloid leukemia
c-myb 5-10-fold acute myeloid
leukemia colon carcinoma c-erbB
30-fold epidermoid carcinoma K-ras
4-20-fold colon carcinoma 30-60-fold adrenoc
ortical carcinoma
19
Tumor suppressor genes Disorders
in which gene is affected Gene (locus)
Function Familial Sporadic
DCC (18q) cell surface
unknown colorectal interactions
cancer WT1 (11p) transcription Wilms
tumor lung cancer Rb1 (13q) transcription
retinoblastoma small-cell lung
carcinoma p53 (17p) transcription
Li-Fraumeni breast, colon, syndrome
lung cancer
20
Sporadic and familial (Mendelian) forms of
cancer Knudsons two-hit hypothesis
Normal tumor suppressor gene
Sporadic
Somatic mutation in one allele
Somatic mutation in other allele
Single tumors, unilateral, later-onset
- two mutations (two hits) are required for loss
of tumor suppressor function
21
Sporadic and familial (Mendelian) forms of
cancer Knudsons two-hit hypothesis
Tumor suppressor gene containing a
germline mutation in one allele - heterozygous
for the mutation
Familial
Somatic mutation in other allele
Multiple tumors, bilateral, early-onset
- two mutations (two hits) are required for loss
of tumor suppressor function - the first hit is inherited and the second
hit is somatic
22
Chromosomal mechanisms that could lead to loss of
function due to loss of heterozygosity
Constitutional genotype
Somatic mutational events - the second hit
Local mutational events
Somatic recombination
Chromosome loss
Chromosome loss and duplication
Tumor genotypes in retinoblastoma
23
Cell-cycle dependent phosphorylation of Rb
Phosphorylation of Rb allows cells to transit the
restriction point and enter S phase
Hyperphosphorylated Rb
p
p
p
p
Rb
Rb
p
p
p
p
Restriction point
S
p
phase
Rb
p
G1
phase
G2
G0
phase
M
Quiescent cells
p
p
Rb
phase
p
p
p
Rb
Hypophosphorylated Rb
p
p
p
Rb
p
p
24
Function of the Rb protein
Growth suppression
Relief of growth suppression
p
p
E2F
Rb
E2F
p
p
G1 phase phosphorylation releases E2F
- E2F is a transcription factor
- that mediates growth-dependent
- activation of genes required to
- make the transition into and
- through S phase
- Rb binds and inactivates E2F
- under conditions of growth
- suppression
- There are several ways to
- alleviate growth suppression
- resulting in controlled or
- uncontrolled cell growth
E2F
E1A
Adenovirus E1A oncoprotein binding releases E2F
p
E2F
Rb
p
Gene mutation affecting binding pocket
releases E2F
25
Li-Fraumeni syndrome - caused by mutations in the
p53 gene
Breast cancer
Sarcoma
Other malignant neoplasms
There are multiple neoplasms in Li-Fraumeni
families that are inherited in an autosomal
dominant fashion
26
- p53 is the guardian of the genome
- germline p53 mutations are found in Li-Fraumeni
syndrome - p53 is frequently found mutated in human tumors
- the p53 protein functions as a transcription
factor that - regulates cell-cycle and DNA repair genes
- UV irradiation causes cell-cycle arrest in G1
that is dependent - on p53 cells that contain a mutated p53 cannot
arrest - and go into S phase and replicate damaged DNA
- p53 loss-of-function mutations result in the
replication of - cells with damaged DNA and to the further
accumulation - of other mutations affecting oncogenes and tumor
- suppressor genes, and to an increased
- likelihood of cancer
27
The role of p53 in the cell cycle
apoptosis (cell death)
DNA synthesis
UV irradiation leads to cell cycle arrest
S
p53
phase
G1
G0
phase
Quiescent cells
G2
phase
Growth and preparation for cell division
M
phase
Mitosis
28
- Learning objectives
- understand the concept of multistep
carcinogenesis and what kinds - of genes are mutated during this process
- understand the differences between oncogenes
and - tumor suppressor genes
- understand the relationships between viral
oncogenes and - host cell oncogenes (proto-oncogenes)
- understand the concept that oncogenes function
in signal transduction - understand the mechanisms by which oncogenes are
activated - during carcinogenesis
- understand the concept that tumor suppressor
genes are lost or - inactivated during carcinogenesis
- understand the concept that a loss of function
mutation can be - expressed as a dominant disease (Knudsons
two-hit hypothesis) - understand the functions of Rb and p53