Thermodynamics Problems PowerPoint PPT Presentations
All Time
Recommended
Chemistry Problems Thermodynamics Blase Ferraris (did these problems for Gangluff) Final Project 1996B and 1997D 1996 B C2H2(g) + 2H2(g) C2H6(g) Information about the ...
| PowerPoint PPT presentation | free to view
Thermodynamics Honors Unit 5
| PowerPoint PPT presentation | free to download
Thermodynamics Chapter 12
| PowerPoint PPT presentation | free to view
What is thermodynamics? Thermodynamics is the study of the effect of work, heat, and energy on a system. System- Motion of particles that determine the state of matter.
| PowerPoint PPT presentation | free to view
Thermodynamics Chapter 5 Thermochemistry Thermochemistry Thermodynamics study of energy and its transformation. Thermochemistry relationship of energy changes ...
| PowerPoint PPT presentation | free to download
Section 1 Relationships Between Heat and Work Chapter 10 Thermodynamics Section 1 Relationships Between Heat and Work Chapter 10 Objectives Recognize that a system ...
| PowerPoint PPT presentation | free to view
If the internal energy of the gas increases by 114 J during the process, what is the total amount of energy transferred as heat?
| PowerPoint PPT presentation | free to view
Heat ~is the total thermal energy of an object. Like all energy, heat is measured in joules (J) The terms hot, warm, cool and cold are always relative.
| PowerPoint PPT presentation | free to download
Thermodynamics is the study of the transformation of energy into heat and for doing work ... and The First Law of Thermodynamics. 1) Energy is conserved ...
| PowerPoint PPT presentation | free to download
Title: No Slide Title Author: Grosse Pointe Public Schools Last modified by: theisej Created Date: 5/28/1995 4:03:36 PM Document presentation format
| PowerPoint PPT presentation | free to view
Otto Cycle Air working fluid; ideal gas Approach: ... 4-stroke internal combustion engine has a bore of 2.55 inches and a stroke of 2.10 inches.
| PowerPoint PPT presentation | free to view
Thermodynamics Begin with a brief review of Chapter 5 Natural systems tend toward states of minimum energy Figure 27.13. P-T pseudosection calculated by THERMOCALC ...
| PowerPoint PPT presentation | free to download
Wind & Heat Loss A breeze can cool us off in the summer, ... Suppose some firewood is brought in from the cold and an apple pie is removed from a hot oven.
| PowerPoint PPT presentation | free to download
Thermal expansion depends on the initial length of the rod. ... All hot objects above absolute zero (0K) emit ELECTROMAGNETIC RADIATION in one ...
| PowerPoint PPT presentation | free to view
Chemical Thermodynamics
| PowerPoint PPT presentation | free to view
Chapter 4 2nd Law of Thermodynamics HW problems Notes: Problems 1-4 Hint for No. 1a: Internal energy has 3 components Chapter 4 2nd Law of Thermodynamics HW problems ...
| PowerPoint PPT presentation | free to download
Thermodynamics Standard 7 Chemistry. Ms. Siddall. Chemical Thermodynamics = the movement of heat in a chemical reaction. Temperature = a measure of the average ...
| PowerPoint PPT presentation | free to download
Thermodynamics Thermodynamics answers the following question: For any reaction - defined by a set of reactants and products set in exactly defined conditions ...
| PowerPoint PPT presentation | free to download
Thermodynamics Meeting 6 Section 4-1 So far we ve studied two forms of energy transfer A note about work and heat: Work and Heat are forms of energy transfers that ...
| PowerPoint PPT presentation | free to download
A lab manual will be available for this course early next week ... http://www.dailymotion.com/cluster/tech/video/xk952_3d-deutz-engine-animati on?from=rss ...
| PowerPoint PPT presentation | free to view
aki kanlar mekan kaynak kitap aki kanlar mekan y.a. engel, jm. cimbala (t rk esi: palme yayincilik *
| PowerPoint PPT presentation | free to view
3-4 isentropic expansion. 4-1 constant-volume heat rejection. ... 1-2 Isentropic compression (in a compressor) 2-3 Constant-pressure heat addition ...
| PowerPoint PPT presentation | free to view
Thermodynamics II. Review Sample Problem. 40.0 g NH4NO3 ... Plot of lnK vs 1/T see back page. PHASE CHANGES - generalizations. To state with more freedom ...
| PowerPoint PPT presentation | free to view
Thermochemistry relationship of energy changes in chemical ... Heat Capacity of an obj (C) is the amt of heat necessary to raise tits tempt by 1 deg C ...
| PowerPoint PPT presentation | free to view
Title: No Slide Title Author: Jeff Joens Last modified by: utsguest Created Date: 8/23/2005 12:05:12 PM Document presentation format: On-screen Show (4:3)
| PowerPoint PPT presentation | free to download
Thermodynamics Assignment Help is a valuable resource for students seeking assistance with their thermodynamics-related assignments and coursework. Thermodynamics is a branch of physics that deals with the study of energy transfer, heat, and the properties of matter at various temperatures. This field can be complex, involving concepts such as entropy, temperature, and energy conservation. https://www.onlinetutorhelps.com/thermodynamics-assignment-help/
| PowerPoint PPT presentation | free to download
Examples of systems that have increased entropy. Entropy increases for: ... 19.4 Entropy Changes in Chemical Reactions. Standard molar entropy values, S (J/mol K) ...
| PowerPoint PPT presentation | free to view
Chapter 12 The Laws of Thermodynamics Answers to the even-numbered problems Chapter 12 Problem 36 6.06 kJ/K Answers to the even-numbered problems Chapter 12 Problem ...
| PowerPoint PPT presentation | free to download
FE Thermodynamics Review Dr. Omar Meza Assistant Professor Department of Mechanical Engineering
| PowerPoint PPT presentation | free to view
... we immediately turned around and introduced elements such as sources and ... leads to additional entropy, which is re-introduced at the nearest 0-junction. ...
| PowerPoint PPT presentation | free to download
Heat and Thermodynamics The Study of Heat Flow and Heat Exchanges Student Activities Pretest Lab work and report Unit test Warm-up Questions Demonstrations and ...
| PowerPoint PPT presentation | free to view
2 points extra credit. Sec 5 Free Energy and Conc. 90. Sec 4 ... such as a gas or a shuffled deck of cards, has relatively large. disorder and high entropy. ...
| PowerPoint PPT presentation | free to view
... car ... plant consumes 80,000 Joules of coal energy to. produce 30,000 Joules of ... What is the efficiency of the car? Heat Pump COP. For heat pumps, it is ...
| PowerPoint PPT presentation | free to view
Given certain changes in props., ( external interactions??). Efficiencies in real processes ... Initial state: N moles at T1, P1. final state: N moles at T2, P2 ...
| PowerPoint PPT presentation | free to view
Thermodynamics of Reactions Spontaneity, Entropy, and Free Energy Chapter 16
| PowerPoint PPT presentation | free to download
Title: THERMOCHEMISTRY or Thermodynamics Author: J. Kotz Last modified by: hisham Created Date: 3/5/1999 5:23:29 PM Document presentation format: A4 Paper (210x297 mm)
| PowerPoint PPT presentation | free to view
Chapter 15 Thermodynamics Thermodynamics States The First Law of Thermodynamics Thermodynamic Processes for an Ideal Gas Reversible and Irreversible Processes Entropy ...
| PowerPoint PPT presentation | free to download
NonEquilibrium Thermodynamics
| PowerPoint PPT presentation | free to view
ENGR 2213 Thermodynamics F. C. Lai School of Aerospace and Mechanical Engineering University of Oklahoma Review Thermodynamic Property Tables of Water Saturated ...
| PowerPoint PPT presentation | free to download
... process Ideal Gas Law: PV=nRT=constant 1st Law: Q=W because U=0 Adiabatic Process: No heat is allowed to flow ... First Law of Thermodynamics ...
| PowerPoint PPT presentation | free to download
Also, UB = UA (isotherm) UA-UC = UB-UC. CP = CV R. Thermal Processes ... Isotherms. PV=nRT. P=nRT/V. Work done by system in isothermal process (T= constant) ...
| PowerPoint PPT presentation | free to view
Chemistry 2402 - Thermodynamics Lecture 12 : Kinetic coefficients and Linear response Lecture 13 : Non Ideal Solutions and Activity Lecture 14: Chemical Equilibria
| PowerPoint PPT presentation | free to download
Chapter 24 Thermodynamics The study of heat and its transformation into mechanical work. If we increase the thermal motion of atoms what happens?
| PowerPoint PPT presentation | free to view
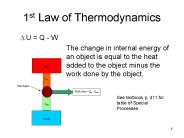
1st Law of Thermodynamics U = Q - W The change in internal energy of an object is equal to the heat added to the object minus the work done by the object.
| PowerPoint PPT presentation | free to download
Chapter 19: Chemical Thermodynamics Tyler Brown Hailey Messenger Shiv Patel Agil Jose Example Cyclopropane and propylene isomers both have the formula C3H6.
| PowerPoint PPT presentation | free to download
v) Boiler: It is an equipment used for the generation of the steam. ... cooled compressor 0.5 kg of air is ... Air enters the compressor at 1 bar and 200C. ...
| PowerPoint PPT presentation | free to view
He used statistical thermodynamics ... Entropy on the Molecular Scale Each thermodynamic state has a specific number of microstates, W, associated with it.
| PowerPoint PPT presentation | free to download
... so that Z1 = f (TR, PR) except near the critical point. ... The pressure can also calculated by reading the value of compressibility factor from the chart. ...
| PowerPoint PPT presentation | free to view
As a basis for understanding this concept: ... Don't Let Your Car's Engine Overheat. Aluminum expands more than iron. Pistons made of aluminum ...
| PowerPoint PPT presentation | free to view
Chemical Thermodynamics Thermochemistry Thermochemistry the part of thermodynamics that involves the relationship between chemical reactions and energy changes I ...
| PowerPoint PPT presentation | free to view
or as thermal diffusion on a washboard potential. Tilting the washboard the other way (e.g. by increasing the PP concentration) ...
| PowerPoint PPT presentation | free to download
... rels ppt/s/_rels/10.xml.rels ppt/s/_rels/16.xml.rels ppt ... xml.rels ppt/s/_rels/18.xml.rels ppt/s/_rels/1.xml.rels ppt ...
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: pratts Last modified by: scott pratt Created Date: 11/2/2002 7:24:45 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to download
Or (Pa Pb) V = (maRa mbRb) T. Also, since the mixture behaves like a perfect gas, ... equation (1) and (2), mm R = maRa mbRb. Also for gas mixture, PaV ...
| PowerPoint PPT presentation | free to view
Suppose our polymer solution was not NBR+acetone, ... Thermodynamic Properties Concerned with macroscopic properties of a body, not atomic properties Volume, ...
| PowerPoint PPT presentation | free to view
Goal: to understand Thermodynamics Objectives: To learn the first law of Thermodynamics To learn about the PV diagram To learn about work done on a gas
| PowerPoint PPT presentation | free to download