1st Law of Thermodynamics - PowerPoint PPT Presentation
Title:
1st Law of Thermodynamics
Description:
1st Law of Thermodynamics U = Q - W The change in internal energy of an object is equal to the heat added to the object minus the work done by the object. – PowerPoint PPT presentation
Number of Views:208
Avg rating:3.0/5.0
Title: 1st Law of Thermodynamics
1
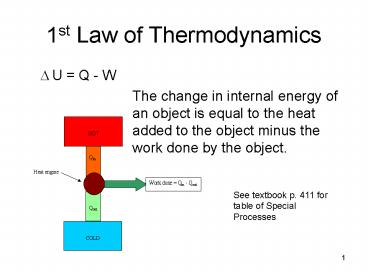
1st Law of Thermodynamics
- U Q - W
- The change in internal energy of an object
is equal to the heat added to the object minus
the work done by the object.
See textbook p. 411 for table of Special Processes
2
Internal energy
?U Q - W
- Isovolumetric Process no change in Volume and
therefore no Work done, so ?U Q - Isothermal Process no change in Temperature and
internal energy, so Q W - Adiabatic Process no energy transferred as
heat, so Q0, so ?U -W - Isolated system no heat or work interaction, so
UiUf
3
A heat engine
- Transforms heat at high temperature into
mechanical energy and low-temperature waste heat - A heat pump (refrigerator) absorbs heat from the
cold reservoir and gives off heat to the hot
reservoir.
4
(No Transcript)
5
(No Transcript)
6
2nd Law of Thermodynamics
- In the 19th Century French engineer Sadi Carnot
studied the ability of engines to convert thermal
energy into mechanical energy. - He developed a logical proof that even an ideal
engine would generate some waste heat. - Carnots result is best described by the term
entropy which is the measure of the disorder in a
system.
7
- The change is entropy, ? S, is shown by the
equation - S Q / T Units J/K
- The change in entropy of an object is equal to
the heat added to the object divided by the
temperature of the object. - Natural processes occur in a direction that
increases the entropy of the universe. All
processes tend toward disorder unless some action
occurs to keep them ordered. i.e., heat can flow
only from hot to cold naturally.
8
Practice Problems
9
12/32 answer
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)