Repulsion PowerPoint PPT Presentations
All Time
Recommended
Title: PowerPoint Presentation Last modified by: rpongchit Created Date: 1/1/1601 12:00:00 AM Document presentation format: On-screen Show (4:3) Other titles
| PowerPoint PPT presentation | free to view
Title: No Slide Title Author: J. David Robertson Last modified by: Mascotti, David P. Created Date: 10/3/1999 7:48:35 PM Document presentation format
| PowerPoint PPT presentation | free to download
Molecular Geometry Molecules of different subtances have diverse shapes. Atoms attach to one another in various geometric arrangements. The overall molecular shape of ...
| PowerPoint PPT presentation | free to download
... to be as far away from one another as possible while remaining in the molecule. Lone pair electrons will spread out more than bonding pair electrons ...
| PowerPoint PPT presentation | free to view
More than one set of bonding pairs of electrons may bind any two atoms together ... Trigonal Bipyramidal. Molecules shaped by five electron pairs ...
| PowerPoint PPT presentation | free to view
Leds are placed to activate the photo detectors each time they enter a torque producing region. ... Simplified positioning of the photo detectors and LEDs ...
| PowerPoint PPT presentation | free to view
... Read the question Choose the correct answer And . . . don t ... Ready to try again? Sorry about that. How about another go round? OOPS! Let s try again, shall ...
| PowerPoint PPT presentation | free to view
An extension of the 'groups of electrons' (effective electron ... sawhorse, see-saw. square. planar. 5. trigonal. bipyramidal. square. pyramidal. 6. octahedral ...
| PowerPoint PPT presentation | free to view
Lone pair-lone pair (LP-LP) repulsion is considered to be stronger than ... Finished? Assign VSEPR shapes to the homework sheet (questions 1-5) from last class. ...
| PowerPoint PPT presentation | free to view
VSEPR THEORY (Valence Shell Electron Pair Repulsion Theory) Adapted by Mr. M. McIsaac Carleton North High School, Bristol, NB From Mr. James Montgomery
| PowerPoint PPT presentation | free to view
5 Trigonal Bipyramid. Molecule Lewis Dot SN EP Structure Molecular Structure ... 5 Trigonal Bipyramid : in axial or equatorial position? E(LPBP) E(BPBP) & Only ...
| PowerPoint PPT presentation | free to download
Gaseous beryllium chloride is an example of a molecule in which the ... Other alkaline earth elements also have the same valence electron ...
| PowerPoint PPT presentation | free to view
Hi-Frequency Common-Mode current paths. 2004 SMMA. 6 ... The adjustable speed brushless repulsion motor eliminates the need for high ...
| PowerPoint PPT presentation | free to view
Times Symbol Blank Valence Shell Electron Pair Repulsion Theory VSEPR Theory VSEPR Theory Draw the Lewis Dot Structure for each of the following Compounds. Split ...
| PowerPoint PPT presentation | free to view
... of AB3 Molecules with No Unshared Electrons on A Trigonal Planar Molecules ... 3 bonding pairs = sp2 hybridized = trigonal planar in shape ...
| PowerPoint PPT presentation | free to view
Competition of Steric Repulsion and Electrostatic Attraction in Model Calcium Channels. Calcium channels conduct Na ions in the absence of Ca2 , but they selectively ...
| PowerPoint PPT presentation | free to view
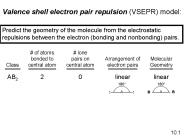
Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and ...
| PowerPoint PPT presentation | free to download
Valence Shell Electron Pair Repulsion. Electron pairs orient themselves so as to make the angles between themselves as large as possible. Repulsion follows Coulomb ...
| PowerPoint PPT presentation | free to download
Chemical Bonding Molecular Shapes (Valence Shell Electron Pair Repulsion) Examples CH4 SF2 NH3 ...
| PowerPoint PPT presentation | free to view
ATOMIC STRUCTURE F is weird -- strong e-/e- repulsion since the p orbitals are really small in the second level, therefore, repulsions are high.
| PowerPoint PPT presentation | free to download
Electrons are transferred from one atom to another creating ( ) & (-) ions. Metal & nonmetal ... Valance Shell Electron Pair Repulsion ...
| PowerPoint PPT presentation | free to download
Colloidal Stability Introduction Interparticle Repulsion Interparticle Attraction Hamaker constant Measurement techniques Solvent Effects Electrostatic Stabilisation
| PowerPoint PPT presentation | free to view
Colloidal Stability Introduction Interparticle Repulsion Interparticle Attraction Hamaker constant Measurement techniques Solvent Effects Electrostatic Stabilisation
| PowerPoint PPT presentation | free to view
Electrons are transferred from one atom to another creating ( ) & (-) ions. Metal & nonmetal ... Valance Shell Electron Pair Repulsion ...
| PowerPoint PPT presentation | free to download
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predicting Molecular ...
| PowerPoint PPT presentation | free to view
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 9 Valence shell electron pair repulsion (VSEPR) model: Predicting Molecular ...
| PowerPoint PPT presentation | free to download
If order is increased, driving force is lost at high temperatures ! ... range 3-4 ?, turns into repulsion at shorter distances ...
| PowerPoint PPT presentation | free to download
Ch. 16 - Magnetism I. Characteristics of Magnets Magnetism Magnetic poles Magnetic field Magnetic domain A. Magnetism Magnetism force of attraction or repulsion ...
| PowerPoint PPT presentation | free to download
THERMODYNAMICS OF SEPARATION OPERATIONS Aseotropes The increased repulsion between molecules can result in the formation of an azeotrope, which is a liquid mixture ...
| PowerPoint PPT presentation | free to download
Playing with. Short Range Attractions and Long Range Repulsions ... Molecular Dynamics Data for Repulsive Yukawa Potential. Several number densities n ...
| PowerPoint PPT presentation | free to download
Ch. 6 Molecular Structure II. Molecular Geometry (p. 183 187) A. VSEPR Theory Valence Shell Electron Pair Repulsion Theory Electron pairs orient themselves in ...
| PowerPoint PPT presentation | free to view
... because they are identically charged, repel each other electrostatically. ... other at speeds high enough (106 m/s) to overcome the electrostatic repulsion. ...
| PowerPoint PPT presentation | free to download
Magnetism Magnetic Poles Magnetic ... these are the areas where magnetic effect is the strongest Always appear in pairs Like Poles = Repulsion Different ...
| PowerPoint PPT presentation | free to download
Prevent agglomeration: electrostatic repulsion and steric exclusion ... Steric exclusion stabilization. Also 'polymeric stabilization' ...
| PowerPoint PPT presentation | free to view
Fatty Acids trans fatty acid cis fatty acid * VSEPR Model The Valence Shell Electron Pair Repulsion model predicts the shapes of molecules and ions by ...
| PowerPoint PPT presentation | free to view
Ch.9.3 Molecular Structure Molecular Geometry A. VSEPR Theory Valence Shell Electron Pair Repulsion Theory Electron pairs orient themselves in order to minimize ...
| PowerPoint PPT presentation | free to view
VSEPR Theory VSEPR Valence Shell Electron Pair Repulsion Provides a 3D representation of molecules which can be used to predict molecular shape Electron orbitals ...
| PowerPoint PPT presentation | free to download
Magnetism What is magnetism? Force of attraction or repulsion due to electron arrangement Magnetic forces are the strongest at the poles Magnets have two poles: North ...
| PowerPoint PPT presentation | free to view
Unit 3E: Magnets and Springs Magnets and Springs T Unit 3E: Vocabulary Useful Words Magnetic Non Magnetic Attraction / Attract Repulsion / Repel Bar Magnet Horseshoe ...
| PowerPoint PPT presentation | free to download
Molecular Geometry NCSCOS Essential Standard Chm 1.2.3 I. VSEPR Theory Valance Shell Electron Pair Repulsion Gives molecules their shape Pairs of electron repel to be ...
| PowerPoint PPT presentation | free to view
Molecular Compounds A. Energy of Bond Formation Potential Energy based on position of an object low PE = high stability no interaction attraction vs. repulsion ...
| PowerPoint PPT presentation | free to view
Magnetism * Natural Magnets ... The true test for determining if something is a magnet is repulsion. A Designer Floating Bed The Earth s Magnetic Field The Earth ...
| PowerPoint PPT presentation | free to download
Chapter 6.5 Quiz 5 Questions 25 points According to VSEPR theory, the electrostatic repulsion between electron pairs surrounding an atom causes an electron sea to ...
| PowerPoint PPT presentation | free to download
Image forces arise for charged particles at regions with big ... Steric repulsion. Lennard Jones potential. Electron distribution in aromatic rings (p- bonding) ...
| PowerPoint PPT presentation | free to download
Eclipsed leads to H-H nuclear repulsion. Repulsion between ... Butane: Gauche and Anti. Butane Rotomers. Cyclopropane. Bond Angles are 60 A lot of Angle Strain ...
| PowerPoint PPT presentation | free to view
The Complementary Shapes of an Enzyme and Its Substrate Valence-shell Electron-Pair Repulsion (VSEPR) ... E = non-bonding electrons (usually a lone pair) ...
| PowerPoint PPT presentation | free to download
Shapes of Molecules All shapes came from http://www.spusd.k12.ca.us/chemmybear/shapes.html VSEPR theory Valence shell electron pair repulsion theory Simply stated it ...
| PowerPoint PPT presentation | free to view
10.3 Valence-Shell Electron-Pair Repulsion (VSEPR) Theory and Molecular Shape 10.4 Molecular Shape and Molecular Polarity Lewis Structures ..
| PowerPoint PPT presentation | free to download
Introduction to Solid State Physics http://www.physics.udel.edu/~bnikolic ... Mott Insulator: A solid in which strong repulsion between the particles impedes ...
| PowerPoint PPT presentation | free to download
The hectic pace of modern lifestyles, combined with a growing repulsion for mass produced goods has sparked a worldwide yearning for greater connection to nature and a deeper appreciation for products that are small batch and handmade. Visit: https://melisperfumery.com/2018/10/30/meet-kristen-watts/
| PowerPoint PPT presentation | free to download
Each element of nature is responsible to structure your body. Earth is responsible for building the solid parts of human body, like bones, tissues, hair, flesh, and skin. Water builds urine, saliva, semen, sweat, and blood. Air regulates every kind of body movements, like expansion, suppression, contraction, etc. Fire forms sleep, hunger, and thirst. Space forms repulsion and physical attraction.
| PowerPoint PPT presentation | free to download
Electron repulsion of orbital lobes determines bonding angles and the resulting molecular shape ... sp3 Hybridized - Tetrahedral shape ...
| PowerPoint PPT presentation | free to view
Magnets and Magnetism 5.19.14 Magnetic repulsion and attraction Magnets act like electrons do A north pole of a magnet will repel the north pole of another magnet.
| PowerPoint PPT presentation | free to download
... (e.g .Watt governor) Maglev train uses magnetic repulsion to reduce friction Module SESA3002a; Aerospace Design James Scanlan; ...
| PowerPoint PPT presentation | free to download
force of attraction or repulsion between unlike or like poles. due to the arrangement of electrons ... in a magnetized object, domains are all aligned. domain ...
| PowerPoint PPT presentation | free to view
Trigonal planar? Tetrahedral? The VSEPR Model. Valence Shell Electron Pair Repulsion Model ... Trigonal Planar. 120o apart. The VSEPR Model. Tetrahedral ...
| PowerPoint PPT presentation | free to view