ClayGashydrate Intercalates - PowerPoint PPT Presentation
1 / 1
Title:
ClayGashydrate Intercalates
Description:
Methane hydrate is abundant in continental shelf and ocean floor sediments, and ... at reduced salinity, clay (smectite) may intercalate CH4 at conditions from ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: ClayGashydrate Intercalates
1
ClayGas-hydrate Intercalates
Stephen Guggenheim, Department of Earth and
Environmental Sciences, University of Illinois at
Chicago
- Methane hydrate is abundant in continental shelf
and ocean floor sediments, and is believed to
form the major reservoir of methane on Earth.
Like ice, methane hydrate is stable at
temperatures near freezing and below, but require
elevated pressures. - New hydrates, claymethane-hydrate intercalates,
were synthesized in a X-ray environmental chamber
at temperatures near 0 oC and CH4 pressures of
25-50 bars. Claymethane-hydrate complexes may
be potentially important in planetary climate
change, because methane is an efficient
greenhouse gas. In addition, they may be
important in energy resource development and in
understanding ocean-floor hazards. The goal of
the project is to determine and understand the
fundamental properties of these new phases.
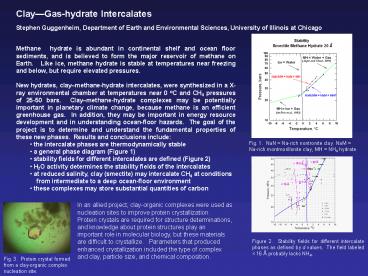
Results and conclusions include. - the intercalate phases are thermodynamically
stable - a general phase diagram (Figure 1)
- stability fields for different intercalates are
defined (Figure 2) - H2O activity determines the stability fields of
the intercalates - at reduced salinity, clay (smectite) may
intercalate CH4 at conditions from
intermediate to a deep ocean-floor environment - these complexes may store substantial quantities
of carbon
Fig. 1. NaN Na-rich nontronite clay. NaM
Na-rich montmorillonite clay, MH NH4 hydrate
In an allied project, clay-organic complexes were
used as nucleation sites to improve protein
crystallization. Protein crystals are required
for structure determinations, and knowledge about
protein structures play an important role in
molecular biology, but these materials are
difficult to crystallize. Parameters that
produced enhanced crystallization included the
type of complex and clay, particle size, and
chemical composition.
Figure 2. Stability fields for different
intercalate phases as defined by d values. The
field labeled lt 16 ? probably lacks NH4.
Fig. 3. Protein crystal formed from a
clay-organic complex nucleation site.