Amine Salts PowerPoint PPT Presentations
All Time
Recommended
In this report, the Global Amine Salts market is valued at USD XX million in 2016 and is expected to reach USD XX million by the end of 2022, growing at a CAGR of XX% between 2016 and 2022.
| PowerPoint PPT presentation | free to download
Amines Ammonia derivatives
| PowerPoint PPT presentation | free to view
Title: AMINA Author: fendi Last modified by: Valued Acer Customer Created Date: 1/16/2005 8:35:41 AM Document presentation format: On-screen Show (4:3)
| PowerPoint PPT presentation | free to download
Whereas the sulfonamide from the secondary amine is insoluble. * * Title: PowerPoint Presentation Last modified by: Sangeeta Mereddy Created Date: 1/1/1601 12:00:00 AM
| PowerPoint PPT presentation | free to download
AMINA Senyawa yang mengandung gugus NH2 Strukrur : RNH2 Jenis : Amina primer (1o) Amina sekunder (2o) Amina tersier (3o) 3 3 6 5 6 5 6 2 2 5 7 2 2 9 3 3 12 12 18 18 ...
| PowerPoint PPT presentation | free to download
Aniline. N-Methylaniline. N-CH3. H. Diphenylamine. H. N. Para-aminomethylbenzene. para-aminotoluene ... H3N: X-R R-NH3 X- ammonia alkyl halide ...
| PowerPoint PPT presentation | free to download
For simple amines, the suffix -amine is added to the name of the ... Nitro Compounds ... nitration of an aromatic compound and reduction of the nitro group ...
| PowerPoint PPT presentation | free to view
Heterocyclic Aliphatic Amines. piperidine. Heterocyclic Aromatic Amines. pyridine. Nomenclature ... 1 and 2 have hydrogen bonding. no hydrogen bonding for 3 ...
| PowerPoint PPT presentation | free to view
Amines with more than one type of alkyl group may be named as ... Many amines have foul odors. Amines are weak bases. Example of biologically active amines ...
| PowerPoint PPT presentation | free to view
aniline. 2-propanamine. isopropylamine. CH3CH(NH2)CH3. 1-propanamine ... N-alkyl'-N-alkyl''aniline. Tertiary amines. N,N-dimethylethanamine. ethyldimethylamine ...
| PowerPoint PPT presentation | free to view
Amines via Reduction. Reduction of nitro compounds. Reduction of nitriles. Reduction of amides ... Amines via Bimolecular Nucleophilic Substitution. Amine Reactions ...
| PowerPoint PPT presentation | free to view
Quaternary Ammonium ion arises when a 4th group is attached to the nitrogen ... The bitterness and poisonous nature of alkaloids probably evolved to protect ...
| PowerPoint PPT presentation | free to view
4.7.0 TYPES OF AMINES. Aromatic amines have the N joined to the benzene ... 4.7.3 MAKING ALIPHATIC 1y AMINES. 4.7.3 MAKING ALIPHATIC 1y AMINES. Butylamine ...
| PowerPoint PPT presentation | free to view
Amines with more than one type of alkyl group may be named as ... amines with three different R groups should be chiral (i.e., have a stereocenter) ...
| PowerPoint PPT presentation | free to view
Amines The organic bases Categorizing Amines Amines are categorized by the number of alkyl groups attached to nitrogen: 1 (primary amine) RNH2 2 (secondary ...
| PowerPoint PPT presentation | free to download
9 Amines IUPAC Name parent chain ...
| PowerPoint PPT presentation | free to view
Amines The organic bases Categorizing Amines Amines are categorized by the number of alkyl groups attached to nitrogen: 1 (primary amine) RNH2 2 (secondary ...
| PowerPoint PPT presentation | free to download
Amines as nucleophiles and their synthesis L.O.: Understand the reaction of amines and ammonia with haloalkanes. Know one application of quaternary ammonium salts.
| PowerPoint PPT presentation | free to view
H-N-H angle 1070 C-N-C angle 1080 ... Physical Properties of Amines. They stink! ... Properties of ammonium salts. High solubility of water ...
| PowerPoint PPT presentation | free to view
1. Structure, nomenclature and physical properties of amines. 2. Acidity and basicity of amines ... Structure, nomenclature and physical properties of amines. 2. ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH3, Nitrogen atom with a ...
| PowerPoint PPT presentation | free to download
Chapter 8 Amines Chapter 19 * Introduction Organic derivatives of ammonia. Many are biologically active. = Additional Amines Biological Activity Neurotransmitters ...
| PowerPoint PPT presentation | free to view
Basicity of Amines Like ammonia, amines are weak bases, ... Aromatic and heterocyclic aromatic. amines are considerably weaker bases than aliphatic amines.
| PowerPoint PPT presentation | free to download
Amines and Amides Chapter 17 Selected biologically important amines Selected biologically important amines Epinepherine (adrenaline): a central nervous system stimulant.
| PowerPoint PPT presentation | free to download
Spectroscopy of Amines - IR Characteristic N H stretching absorptions 3300 to 3500 cm-1. NH2 group shows an irregular doublet, NH - weak multiple bands.
| PowerPoint PPT presentation | free to view
Alkaloids are amines produced by plants. ... Amine salts are named by naming the positive ion first and then naming the negative ion. Properties of Amine Salts ...
| PowerPoint PPT presentation | free to view
Primary amines are named in systematic (IUPAC) nomenclature by replacing the -e ... Reaction of Primary Aliphatic Amines with Nitrous Acid ...
| PowerPoint PPT presentation | free to download
... the more highly substituted alkene product predominates in the E2 reaction of an ... the less highly substituted alkene predominates in the Hofmann elimination due ...
| PowerPoint PPT presentation | free to view
Aliphatic amines. N bonded to alkyl groups. Aromatic Amines. N bonded to one or ... Heterocyclic aliphatic amines. Ring is saturated and N is part of a ring ...
| PowerPoint PPT presentation | free to view
primary amine. (N is a locant-it is not alphabetized, but ... Lone pair on amine nitrogen is conjugated with p-nitro group more delocalized ...
| PowerPoint PPT presentation | free to view
Amines Chemical / Biological / Neurological Activity
| PowerPoint PPT presentation | free to view
Aryl diazonium salts are stable at 0 C. Alkyl diazonium salts generally are not useful. ... Coupling Reactions of Aryl. Diazonium Compounds. Example: Mechanism: ...
| PowerPoint PPT presentation | free to view
Based on McMurry s ... the compound is designated as being heterocyclic Each ring system has its own parent name 24.2 Structure and Bonding in Amines Bonding ...
| PowerPoint PPT presentation | free to view
Chapter 24 Amines and Heterocycles John E. McMurry www.cengage.com/chemistry/mcmurry Paul D. Adams University of Arkansas Heating an acyl azide prepared from an ...
| PowerPoint PPT presentation | free to view
BIOGENIC AMINES PRODUCED BY MICROORGANISM Minggu-3 B.A. : Histamine, Tyrramine, Tryptamine, Cadavarine, Putrescine, 2-Phenyl-ethylamine, Spermidine, and Spemine ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH3, Nitrogen atom with a ...
| PowerPoint PPT presentation | free to view
2-methyl-1-butylamine. 3-pentanamine. 3-methyl-3-pentanamine. Fig. 17.1. Classification of amines is related to the number of R groups attached to the nitrogen atom. ...
| PowerPoint PPT presentation | free to view
Amine Oxide Market is forecast to reach $564 million by 2026, after growing at a CAGR of 3.6% during 2021-2026. Amine oxides, which are also known as amine-N-oxide and N-oxide, are mild surfactants and is thus considered to be the main factor driving the market for amine oxides in skin-friendly personal care products.
| PowerPoint PPT presentation | free to download
Acids, Bases, Salts, Solubility, And stuff like that! 6.12. NAMES OF ACIDS, BASES, AND SALTS Acids H and a nonmetal: Hydro-ic acid Hydrochloric acid, HCl Oxygen ...
| PowerPoint PPT presentation | free to view
Chemical properties of Amines ... Chemical properties of Aldehydes and Ketones. 1. Oxidation: only for aldehydes (no ketones) ...
| PowerPoint PPT presentation | free to view
Amines are compounds that contain one or more organic groups bonded to nitrogen. ... shared with another atom forming a 'dative' covalent bond (so called because the ...
| PowerPoint PPT presentation | free to view
aliphatic amine ?????????????? ammonia ????? inductive effect ????? cation ????????????????? ... aromatic amine ??????????????? aliphatic amine ????? resonance effect ...
| PowerPoint PPT presentation | free to download
1. common - name the alkyl portions and. follow this by the suffix ' ... deactivating group. Chapter 19 -THE END. 19. To get around this we normally use an amide: ...
| PowerPoint PPT presentation | free to view
Alkyl-substituted (alkylamines) or aryl ... Reduction Aryl Nitro Compounds ... prepared from nitration of an aromatic compound and reduction of the nitro group ...
| PowerPoint PPT presentation | free to view
* Focus on the Human Body Epinephrine and Related Compounds A hormone is a ... Amines Structure and Classification Amines are organic nitrogen compounds, ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines and Heterocycles Based on McMurry s Organic Chemistry, 7th edition * Hofmann Rearrangement RCONH2 reacts with Br2 and base Gives high yields of ...
| PowerPoint PPT presentation | free to view
Amines are derivatives of ammonia, NH3, where one or more hydrogen atoms have ... Name by changing 'amine' to 'ammonium' and adding the anion name. ...
| PowerPoint PPT presentation | free to view
Title: Amino Acids Proteins, and Enzymes Author: Timberlake Last modified by: Rubin Created Date: 8/20/2000 11:52:07 PM Document presentation format
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Author: Timberlake Last modified by: Sarah Garcia Created Date: 8/17/2000 7:29:57 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to download
Chapter 11 Reactions of Alcohols, Ethers, Epoxides, Amines, and Thiols Paula Yurkanis Bruice University of California, Santa Barbara Protonating an Amine Does Not ...
| PowerPoint PPT presentation | free to view
Naturally occurring amino acids has an amino group (NH2) to the carboxyl group (COOH) ... Since it exists as internal salt, known as zwitterion, ...
| PowerPoint PPT presentation | free to view
secondary, and tertiary amine is only 0.3 pK units. Effect of Structure on Basicity ... Lone pair on amine nitrogen is conjugated with p-nitro group more delocalized ...
| PowerPoint PPT presentation | free to download
Nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic ... Chirality Is Possible (But Not Observed) ...
| PowerPoint PPT presentation | free to view
Biomolecules: Amino Acids, Peptides, and Proteins Based on McMurry s Organic Chemistry, 6th edition Proteins Amides from Amino Acids Amino acids contain a basic ...
| PowerPoint PPT presentation | free to view
What is the functional group for this class of organic molecules? ... N-H bonds not as electronegative as O-H bonds, less intermolecular forces. Solubility ...
| PowerPoint PPT presentation | free to view
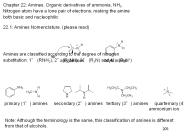
Amines are classified according to the degree of nitrogen ... 22.3: Physical Properties. ( please read) 22.4: Basicity of Amines. ...
| PowerPoint PPT presentation | free to download