Amine Salt Formation PowerPoint PPT Presentations
All Time
Recommended
In this report, the Global Amine Salts market is valued at USD XX million in 2016 and is expected to reach USD XX million by the end of 2022, growing at a CAGR of XX% between 2016 and 2022.
| PowerPoint PPT presentation | free to download
Amines Ammonia derivatives
| PowerPoint PPT presentation | free to view
Title: AMINA Author: fendi Last modified by: Valued Acer Customer Created Date: 1/16/2005 8:35:41 AM Document presentation format: On-screen Show (4:3)
| PowerPoint PPT presentation | free to download
AMINA Senyawa yang mengandung gugus NH2 Strukrur : RNH2 Jenis : Amina primer (1o) Amina sekunder (2o) Amina tersier (3o) 3 3 6 5 6 5 6 2 2 5 7 2 2 9 3 3 12 12 18 18 ...
| PowerPoint PPT presentation | free to download
For simple amines, the suffix -amine is added to the name of the ... Nitro Compounds ... nitration of an aromatic compound and reduction of the nitro group ...
| PowerPoint PPT presentation | free to view
Amines via Reduction. Reduction of nitro compounds. Reduction of nitriles. Reduction of amides ... Amines via Bimolecular Nucleophilic Substitution. Amine Reactions ...
| PowerPoint PPT presentation | free to view
Amines as nucleophiles and their synthesis L.O.: Understand the reaction of amines and ammonia with haloalkanes. Know one application of quaternary ammonium salts.
| PowerPoint PPT presentation | free to view
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH3, Nitrogen atom with a ...
| PowerPoint PPT presentation | free to download
Amines and Amides Chapter 17 Selected biologically important amines Selected biologically important amines Epinepherine (adrenaline): a central nervous system stimulant.
| PowerPoint PPT presentation | free to download
Naturally occurring amino acids has an amino group (NH2) to the carboxyl group (COOH) ... Since it exists as internal salt, known as zwitterion, ...
| PowerPoint PPT presentation | free to view
Alkaloids are amines produced by plants. ... Amine salts are named by naming the positive ion first and then naming the negative ion. Properties of Amine Salts ...
| PowerPoint PPT presentation | free to view
Primary amines are named in systematic (IUPAC) nomenclature by replacing the -e ... Reaction of Primary Aliphatic Amines with Nitrous Acid ...
| PowerPoint PPT presentation | free to download
Non-standard amino acids. Formation of Peptide Bonds. The building blocks of proteins ... Amphoteric. Amino group is protonated. Carboxyl group is deprotonated ...
| PowerPoint PPT presentation | free to view
... the more highly substituted alkene product predominates in the E2 reaction of an ... the less highly substituted alkene predominates in the Hofmann elimination due ...
| PowerPoint PPT presentation | free to view
Amines Chemical / Biological / Neurological Activity
| PowerPoint PPT presentation | free to view
Proteins / polypeptides - chains formed by the condensation ... give permanent structure (recall : position of the disulfide ... Peanut Butter ...
| PowerPoint PPT presentation | free to view
Chapter 24 Amines and Heterocycles John E. McMurry www.cengage.com/chemistry/mcmurry Paul D. Adams University of Arkansas Heating an acyl azide prepared from an ...
| PowerPoint PPT presentation | free to view
BIOGENIC AMINES PRODUCED BY MICROORGANISM Minggu-3 B.A. : Histamine, Tyrramine, Tryptamine, Cadavarine, Putrescine, 2-Phenyl-ethylamine, Spermidine, and Spemine ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH3, Nitrogen atom with a ...
| PowerPoint PPT presentation | free to view
This PowerPoint presentation is talking all about the 5 useful methods of AMINO ACIDS, which help the students to gain knowledge about the AMINO ACIDS assignment. If you need help with your AMINO ACIDS assignment, visit our website: https://www.assignments4u.com/amino-acids-assignment-help/
| PowerPoint PPT presentation | free to download
Amines are compounds that contain one or more organic groups bonded to nitrogen. ... shared with another atom forming a 'dative' covalent bond (so called because the ...
| PowerPoint PPT presentation | free to view
1. common - name the alkyl portions and. follow this by the suffix ' ... deactivating group. Chapter 19 -THE END. 19. To get around this we normally use an amide: ...
| PowerPoint PPT presentation | free to view
Alkyl-substituted (alkylamines) or aryl ... Reduction Aryl Nitro Compounds ... prepared from nitration of an aromatic compound and reduction of the nitro group ...
| PowerPoint PPT presentation | free to view
* Focus on the Human Body Epinephrine and Related Compounds A hormone is a ... Amines Structure and Classification Amines are organic nitrogen compounds, ...
| PowerPoint PPT presentation | free to view
the structures of the side chains of all 20 natural amino acids ... The protein is put in a bag of cellulose membranes having small pores of controlled size. ...
| PowerPoint PPT presentation | free to view
Chapter 24. Amines and Heterocycles Based on McMurry s Organic Chemistry, 7th edition * Hofmann Rearrangement RCONH2 reacts with Br2 and base Gives high yields of ...
| PowerPoint PPT presentation | free to view
Amines are derivatives of ammonia, NH3, where one or more hydrogen atoms have ... Name by changing 'amine' to 'ammonium' and adding the anion name. ...
| PowerPoint PPT presentation | free to view
Title: Amino Acids Proteins, and Enzymes Author: Timberlake Last modified by: Rubin Created Date: 8/20/2000 11:52:07 PM Document presentation format
| PowerPoint PPT presentation | free to download
Synthesis of Single Enantiomers How do chemists achieve the synthesis of single ... Synthesis of Single Enantiomers In a second strategy, asymmetric induction, ...
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Author: Timberlake Last modified by: Sarah Garcia Created Date: 8/17/2000 7:29:57 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to download
Chapter 11 Reactions of Alcohols, Ethers, Epoxides, Amines, and Thiols Paula Yurkanis Bruice University of California, Santa Barbara Protonating an Amine Does Not ...
| PowerPoint PPT presentation | free to view
Nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic ... Chirality Is Possible (But Not Observed) ...
| PowerPoint PPT presentation | free to view
Biomolecules: Amino Acids, Peptides, and Proteins Based on McMurry s Organic Chemistry, 6th edition Proteins Amides from Amino Acids Amino acids contain a basic ...
| PowerPoint PPT presentation | free to view
1. Draw a chemical mechanism for the reduction of a disulfide ... Elution: salt (NaCl, KCl) high to low. A: /- B: neutral, hydrophobic. A elutes first, ...
| PowerPoint PPT presentation | free to view
Since we only have a weak nucleophile and a poor electrophile we need to activate the amide. Protonation of the amide carbonyl makes it more electrophilic. 2. ...
| PowerPoint PPT presentation | free to view
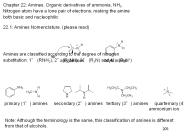
Amines are classified according to the degree of nitrogen ... 22.3: Physical Properties. ( please read) 22.4: Basicity of Amines. ...
| PowerPoint PPT presentation | free to download
Reduction nitroarenes in alkaline medium ... CH3 CH2 NO2 + 3H2 CH3 CH2 NH2 + 2H2O 4.The aromatic nitrocompounds The simplest aromatic nitro compound, ...
| PowerPoint PPT presentation | free to view
Flavor/Odor. Raspberries. HCOOCH2CH3 ethyl methanoate (IUPAC) ethyl formate (common) ... compound, which is responsible for the flavor and odor of pears. ...
| PowerPoint PPT presentation | free to view
Step 4: Formation of arginine and fumarate from arginosuccinate. Argininosuccinase ... Step 5: Hydrolysis of arginine to form ornithine and urea. Ornithine ...
| PowerPoint PPT presentation | free to view
Nucleophilic ring opening of epoxides by ammonia (Section 16.12) ... As octylamine is formed, it competes with ammonia for the remaining 1-bromooctane. ...
| PowerPoint PPT presentation | free to download
22-2. Preparation of Amines. Two questions to answer: 1) How is the C N bond to be formed? 2) How do we obtain the correct oxidation state of nitrogen (and carbon) ...
| PowerPoint PPT presentation | free to view
other AAs are formed by a posttranslational modification ... amphoteric electrolytes) 'AMPHION' Important reactions of AAs. dissociation (formation of salts) ...
| PowerPoint PPT presentation | free to view
Yoghurt pots, refrigerator linings, vending cups, bathroom cabinets, toilet ... Polyamides (examples Nylons, Twaron, Kevlar) formation. acid chlorides with amines ...
| PowerPoint PPT presentation | free to view
Alkaloids exhibiting basic character are very much sensitive to decomposition & cause a problem during storage their salt formation. The alkaloids may contain one ...
| PowerPoint PPT presentation | free to view
Use acetic formic anhydride to produce formate esters and formamides. ... Lactam Formation. Five- and six-membered rings can be formed by heating - and -amino acids. ...
| PowerPoint PPT presentation | free to view
Chapter 20 Amines Ch. 20 - * ...
| PowerPoint PPT presentation | free to download
Food Safety & Toxicology (II) Toxic Microbial Metabolites 1. Biogenic amines (biogenic substance with an amine group) The main producers of biogenic amines in foods ...
| PowerPoint PPT presentation | free to download
Nitrogen compounds (Chapter 37) Nitrogen containing compound T.N.T. Proteins Enzymes Drugs Amines Aliphatic 1o amine 2o amine 3o amine 4o ammonium cpds Aromatic IUPAC ...
| PowerPoint PPT presentation | free to view
CHE-302 Review Diazonium salts synthesis benzenediazonium ion Diazonium salts, reactions Coupling to form azo dyes Replacements a) -Br, -Cl, -CN b) -I c) -F d) -OH ...
| PowerPoint PPT presentation | free to download
(a) Primary aliphatic amines ... 2o aliphatic amines. Form a mixture of alkenes, alcohols, alkyl halides and nitrogen gas. ...
| PowerPoint PPT presentation | free to view
Aliphatic and aromatic groups. Amine groups. Structure of a generic ... Aliphatic ... quinones), methoxyl (ROCH3), aliphatic, aromatic, and amine ...
| PowerPoint PPT presentation | free to view
nitrosation of secondary amines gives an N-nitroso amine. Dr. Wolf's CHM 201 & 202 ... nitrosation of a primary alkylamine gives an alkyl diazonium ion ...
| PowerPoint PPT presentation | free to view
Reaction of amines with Nitrous Acid. Primary Amines (R-NH2) react with nitrous ... Rxns occur under milder conditions ... Milder reaction conditions required ...
| PowerPoint PPT presentation | free to view
1-3(methyl, dimethyl trimethy) are gases (aliphatic only) ... RNH2 ArNH2 aliphatic more basic than aromatic - Amine RCONH2 (Amide) less basic from amine ...
| PowerPoint PPT presentation | free to view
Nitrogen-Containing Compounds The amines and amides are the two major classes of nitrogen-containing compounds. Amines isolated from plants form a group of compounds ...
| PowerPoint PPT presentation | free to view
But not all acid base reactions involve water, and many bases (NH3, carbonates) ... Aromatic amines are much weaker bases than aliphatic amines. ...
| PowerPoint PPT presentation | free to view