XRD XRay Diffraction - PowerPoint PPT Presentation
1 / 20
Title:
XRD XRay Diffraction
Description:
X-rays are generated by striking a target material with an accelerated e- which ... an infinite number of planes can exist, but certain ones diffract more strongly ... – PowerPoint PPT presentation
Number of Views:616
Avg rating:3.0/5.0
Title: XRD XRay Diffraction
1
XRD X-Ray Diffraction
2
X-rays
- Another part of the electromagnetic spectrum
between 100 and 0.2 Å. - Plancks law Ehn hc/l
- Where n is frequency, l is wavelength, h is
Plancks constant, and c is the speed of light
3
X-ray generation
- X-rays are generated by striking a target
material with an accelerated e- which causes an
excitation. When his excitation relaxes, or
goes back down to standard state, an X-ray is
emitted - Usually given in terms of the energy levels those
e- come from and go to ? different levels yield
X-rays of different energies (all dependent on
the material) - K, L, M shells of a material, from that those
shells have different transitions and
characteristic relaxations (a, b, g) - Cu Ka is the most intense peak and most commonly
used (though others are possible and have a
different wavelength, which can be useful!)
4
X-Ray interaction
- Scattering oscillation of incoming X-rays
transfer energy to electrons in material,
emitting secondary radiation at about the same
frequency and energy as the incoming beam - Interaction of X-rays with same material causes
some electrons to go into an excited state, which
upon relaxation, emits radiation characteristic
of the atom it excited ? basis for XRF, used to
identify chemical makeup of materials - As with other interactions with minerals, there
can also be reflection and transmission of X-rays
(depending on thickness), but we dont typically
use that information.
5
Interference
- Constructive and destructive interference wave
properties interact to either cancel out or
amplify each other. - When 2 centers are emitting energy at some
wavelength, they will interfere with each other
Plane view
6
Experiment
- Relationship between light as particles vs. light
as waves - Light scattered by mesh - as it travels and
interacts, some waves compliment each other while
different waves cancel each other
7
Diffraction
- Combine elements of interference with striking
the x-ray at an angle to the material - Relationship between wavelength, atomic spacing,
and angle of diffraction for 3-D structures
derived by von Laue - Braggs determined that you could simplify this
and treat it as a reflection off of the planes
within an atom
8
Braggs Law
- nl2dsinT
- Where n is the order of diffraction (always an
integer), l is the wavelength of incident
radiation, d is the spacing between planes, and T
is the angle of incidence (or angle of
reflection, they are equal)
9
Diffraction
- Relationship between diffraction and wavelength
- The smaller the diffracting object, the greater
the angular spacing of the diffraction pattern - i.e. the smaller the separation between planes,
the wider the spacing between diffraction lines - What then is diffraction??
- The failure of light to travel in straight lines
(much to Newtons dismay) - Youngs 2 slit experiment proved light could bend
scattered and affected by constructive and
destructive interference - Bright red constructive dark destructive
10
Braggs Law
- nl2dsinT
- Where n is the order of diffraction (always an
integer), l is the wavelength of incident
radiation, d is the spacing between planes, and T
is the angle of incidence (or angle of
reflection, they are equal) - Diffraction here is between parallel planes of
atoms ? the space between them (d) determines the
angle of diffraction. - Looking at the laser pattern again ? where is
Braggs Law satisfied and how many orders of
diffraction do we see?
11
Red Laser analogue
- We see orders of diffraction resulting from light
coming between grid spacing 2, 3, 4, 5, etc.,
apart. In a mineral, multiple parallel planes
yields similar patterns at higher orders of
diffraction theoretically the angle keeps
increasing ? what do we notice about the
intensity though?
12
Braggs Law
- nl2dsinT
- Just needs some satisfaction!!
13
X-Ray Diffraction (XRD) equipment
XRD machines vary angle as 2T because that angle
is always relative to incident X-ray beam
trajectory
- nl2dsinT
- nl/2dsinT
- Solution satisfied at specific angles (n MUST
be an integer)
2T
14
XRD Part II
- Theoretically, almost an infinite number of
planes can exist, but certain ones diffract more
strongly - Related to the atomic density both of of
atoms and in those ions atomic density
15
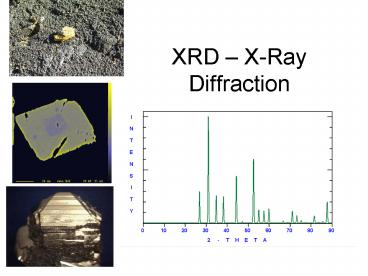
XRD results
- Diffraction pattern
- Higher symmetry ? fewer, more intense lines
because multiple planes are complimentary
(identical d-spacings for different planes yields
identical diffraction)
16
pyrite
fayalite
17
XRD extinctions
- Some forms exhibit extinctions when planes
should be present (i.e. satisfy Braggs Law) but
are not due to destructive interference with
another planes diffraction. - Useful for determining special conditions of
symmetry in a single crystal ID for body, face
centered minerals as well as ones with screw axes
and glide planes ? method to see differences
between space groups
18
XRD analyses
- Can look at minerals as single crystals or as a
powder - Single Crystal ? must be careful about orienting
the crystal so Braggs Law is satisfied, use
several different techniques, advanced machines
manipulate the sample in 3 axes (x,y,z) to
catch all the peaks ? required for structural
determination - Powder has many particles with planes at many
different orientations ? many orientations
satisfy Braggs Law, intensities and locations
(2T) are characteristic of specific minerals.
Technique primarily used for identification
19
Powder XRD analyses
- With a single crystal, alignment of planes which
give strong diffraction returns is very exact
requires precise alignment - With a fine powder, idea is to have crystals at a
wide variety of orientations so hitting that
exact alignment is possible without manipulating
the sample i.e. in a powder we figure a few
grains are lined up correctly
20
Powder X-ray Analyses
- XRD analysis of a powder is a common, quick, and
relatively easy way to identify minerals. - Having a mixture of minerals can be tricky, so
grains are first separated if possible (small
amounts of other minerals will give other peaks,
but intensities are low enough that it is not a
big deal) - Do lose the ability to see the details of the
structure of the mineral however as the precise
alignment of the mineral giving the peak is
unknown and not changeable