Principles of Flow Cytometry - PowerPoint PPT Presentation
1 / 57
Title:
Principles of Flow Cytometry
Description:
This primitive diagram shows the principle: Cells are passing the ... Coulter FC500. BD Diva. Others. Post-acquisition software. FCS Express. FCS Press. WinList ... – PowerPoint PPT presentation
Number of Views:736
Avg rating:3.0/5.0
Title: Principles of Flow Cytometry
1
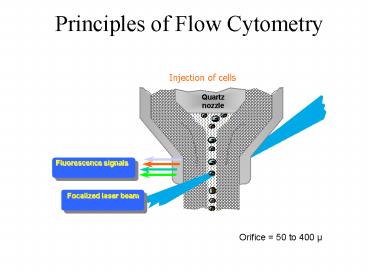
Principles of Flow Cytometry
Injection of cells
Orifice 50 to 400 µ
2
Light can be measured at 90 Side scatter
Fluorescence
Laser
Side scatter reflects the cell content
3
Fluorescence intensity
Number of Events
101
104
103
102
Relative fluorescence intensity
4
Basics of Flow Cytometry
- Cells in suspension
- flow in single-file through
- an illuminated volume where they
- scatter light and emit fluorescence
- that is collected, filtered and
- converted to digital values
- that are stored on a computer
Fluidics
Optics
Electronics
5
The automated Microscope
Detector Counter
This primitive diagram shows the principle Cells
are passing the microscope objective, and an
electronic circuit decides whether the cells is
fluorescent or not. This is how a flow cytometer
works!
Waste
Sample
6
Hydrodynamic focussing in the cuvette
Sample
Sample
Sheath
Sheath
Sample pressure low, small core stream. Good for
DNA analysis
High sample pressure, broader core stream. Bad
for DNA analysis
1
7
Summary
- Pressure ( Sheath Pressure) drives the sheath
buffer through the cuvette, and the higher
pressure in the sample tube( Sample
Differential) delivers the sample to the cuvette. - In the cuvette the principle of hydrodynamic
focussing arranges the cells like pearls on a
string before they arrive at the laser
interception point for analysis - Hydrodynamic focussing cannot separate cell
aggregates! Flow cytrometry is a technique that
requires single cell suspensions
8
Basic opticsc
A system of prisms and lenses directs the laser
light to the interrogation point in the cuvette
9
Laser delay
- Umonuje cross beam kompenzaci
- Vyaduje stabilní fluidics
10
Summary
- Excitation light is steered with prisms and
lenses to the interception point - Emitted light is collected using lenses and is
split up with dichroic mirrors and filters
11
Tasks for the electronical system
- Convert the optical signals into electonic
signals (voltage pulses) - Digitise the data
- Analyse Height (H), Width (W) and Area (A) of the
pulse - Send the data to the analysis computer
12
How a voltage pulse from the PMT is generated
Voltage
13
Height, Area, and Width
Pulse area(A)
Pulse Height (H)
Voltage
40
0
Time (µs)
Pulse Width (W)
14
Threshold
The threshold defines the minimal signal
intensity which has to be surpassed on a certain
channel. All signals with a lower intensity are
not displayed and not recorded for later analysis.
15
Summary
- During passing the laser voltage pulses are
generated at the PMT - Amplifiers enhance the signals
- Only signals passing the desired threshold(s) are
analysed and recorded - The data are finally passed to the analysis
computer connected to the cytometer
16
An overview
Instrument Introduced
Most Frequently Heard Comments
Year
17
Why always more colours?
- More informations from Cell Phenotyping (Cell
Surface Antigens) - around 300 CD Cell Surface Antigens
- Many functional populations require 5 or more
surface markers to be fully distinguished - Functional Assays
- Cell Cycle (PI, BrdU, Intracellular Cyclins)
- Apoptosis (Annexin-V, Active Caspase-3)
- Ca Flux Indo-1, FuraRed, Fluoro-4
- Cytokine Production
- Intracellular Signaling (Rb phosphorylation)
- Gene Reporter Molecular Assays
- GFP, BFP, YFP, CFP Expression
- LacZ Expression
18
(No Transcript)
19
(No Transcript)
20
(No Transcript)
21
What are the advantages / disadvantages?
- Advantages
- Save Time and Samples
- (1) 6-color stain (15) 2-color stains
- Exponential increase in information
- Data from (1) 6-color stain (15) 2-color
stains - Identify new/rare populations (lt0.05)
- Internal controls
- Problems
- Must carefully choose combinations of
fluorochrome conjugates - Not all reagents are available in all colors
- Greater potential for errors in compensation
- Proper controls required
22
Excitation- and Emission spectra of dyes for the
blue laser
- Stejná excitace ruzná emise
- Prekryv spekter
- (overlap)
- Excitace jiným laserem?
23
Compensation
www.bdbiosciences.com/spectra /
24
How much compensation is correct?
PE
PE
25
Importance of ACCURATE Compensation
n negatives d dim positives b bright
positives
26
Which marker for compensation?
Small errors in compensation of a dim control (A)
can result in large compensation errors with
bright reagents (B C). Use bright markers to
setup proper compensation.
27
Hardware Compensation
- How to set compensation on the instrument
28
Setting compensation
- Prepare single stained controls that have both a
positive and negative population. - Adjust the PMT voltages so that the negative
population is off the axis in every channel. - Align the centers of the positive and negative
cell populations by matching the median
fluorescence.
29
Setting compensation- PMT Voltage
-Run unstained cells -Adjust the PMT voltages so
that the negative population is off the axis
in every channel.
FL2-no stain
FL1-no stain
30
Setting compensation - FITC single stain
-Run single stained control (FITC stained
only) -Adjust the compensation value so that
positive and negative population have the same
FL2 median fluorescence intensity.
Uncompensated
Compensated
FL2-no stain
FL2-no stain
Median values both 3.2
FL1-FITC CD3
FL1-FITC CD3
31
Setting compensation - PE single stain
-Run single stained control (PE stained
only) -Adjust the compensation value so that
positive and negative population have the same
FL1 median fluorescence intensity.
Compensated
FL2-PE CD4
Median values both 2.5
FL1-no stain
32
Compensation Controls
- Single Stain Controls
33
Single Stain Controls - Which cells?
- Does not matter as long as
- The autofluorescence is the same in the negative
and positive populations you are lining up. - eg, Pre-gate on lymphocytes if you are using CD8
FITC as a single stain control - The compensation values will be valid for ALL
cell types, regardless of which type of cell is
used to calculate the values. - The compensation is specific for the
fluorochrome, not the cell type
34
Single Stain Controls - which reagents?
- Use the same reagent (Ab-fluorochrome conjugate)
as used in the experimental sample - OR
- A different antibody may be substituted, as long
as it is conjugated to the same fluorochrome. - However
35
Single Stain Controls - which reagents?
- Caveats for substituting reagents
- Controls should be as bright as possible
- As bright or brighter than the experimental
stains - GFP, CFSE, and FITC are NOT the same fluorochrome
- even though they are all green!
- With tandem dyes (Cy5PE/Cy7PE etc.) it is
necessary to use the exact same reagent - spillover varies from reagent to reagent
36
Compensation of tandem-conjugates can differ from
lot to lot
37
Using Antibody Capture Beads as single stained
controls
- Use same reagent as experimental sample
- Lots positive
- Small CV, bright
- Some reagents wont work (IgL, EMA/PI)
- can mix with regular comps
38
Software Compensation
- Automated Tools for Setting Compensation
39
Compensation Tools
- Must have single stained controls
- Software calculated compensation for you!
- Easy, accurate and quick.
- Makes MULTI- Color compensation possible
40
Software Compensation Tools
- Available on new generation machines
- DakoCytomations Summit (version 4)
- Coulter FC500
- BD Diva
- Others
- Post-acquisition software
- FCS Express
- FCS Press
- WinList
- FlowJo
- Others
41
Compensation Matrix
42
Compensation - Automatic Method
- Automatic compensation in the Diva software
offers a fast, easy and reliable method to set
the correct compensation. - First, select Create Compensation Tubes from
the Instrument Menu
43
Compensation - Automatic Method
The software automatically creates a list of
single color tubes, based on your instrument
setting. Naturally, in certain experiments you
may not want to use every channel for a
multicolour experiment. You can delete any tube
from this list, but the corresponding channel is
later, after automatic compensation, also deleted
from your instrument setting!
44
Take Away Lessons
- Proper CONTROLS are essential
- DONT compensate by eye
- Use Median to adjust the populations if you must
do it manually - TRUST the software to do it for you
- It does it quicker and more accurately
45
Polychromatická cytometrie
Design experimentu a analýza
Ústav imunologie, Klinika detské hematologie a
onkologie, UK 2.LF a FN Motol Praha
Childhood Leukemia Investigation Prague -
46
Which fluorochrome to use?
- Major Factors
- Fluorochrome brightness
- PerCP APC-Cy7 FITC ltlt PerCP-Cy5.5 lt PE-Cy7
lt APC PE-Cy5 lt PE - Antigen density
- Background staining of mAb
- Inherent background (stickiness) of mAb
- Antibody strength (Avidity)
- Less antibody needed less background
- Amount of compensation required between
conjugates - Single or multiple laser
47
Comparison of the dye intensity for the same
marker
Baumgarth, Roederer, JIM, 2000, A practical
approach to multicolor flow cytometry for
immunophenotyping
48
Spektra fluorochromu
www.bdbiosciences.com/spectra /
49
Which fluorochrome for which marker?
- In general, try to use brighter fluorochrome
conjugates for duller antibodies or lower density
antigens (e.g. activation antigens such as CD80,
CD86, CD25, or CD28) - Use brighter reagents for staining cell
populations with high autofluorescent backgrounds
(e.g. granulocytes, monocytes, or activated
lymphocytes) - Use duller conjugates (FITC or PerCP) for
antigens expressed at high levels (e.g. B220 or
CD4)
50
Zkreslení vlivem kompenzací
Presvit (spilover, spectral overlap) z PE do FITC
je malý malá kompenzace z PE do PE-TxRed je
velký velká komp.
FITC
PE
PE-TxRed
PE
51
Zkreslení vlivem kompenzací
Je treba promyslet odkud se dívat
Obvyklé problémy PE vs PE-TxRed, PE-Cy5 vs APC
Nelze pouít vdy histogram
Nelze vdy pouít ctverce ci kvadranty
Je treba promyslet jak postavit gate (kontroly
FMO)
V silne komp. kanálech je mení rozliení a horí
kvantifikace
52
Design experimentu
Na interpretaci dat myslet PREDEM
Bez správných kontrol nekdy interpretovat nelze
Jak naloit se zkreslením komp. dat?
Sestavit design experimentu tak, aby se predelo
potíím pri analýze
53
Jak naloit se zkreslením?
PE ? PE-TxRed
donor
akceptor
Na donor pozitivních bunkách se akceptor
pozitivní znak nevyskytuje/nehodnotí
- u CD4 PE pos. bunek CD8 PE-TxRed není
CD8 PE-TxRed
CD4 PE
54
Jak naloit se zkreslením?
PE ? PE-TxRed
donor
akceptor
- znak s nízkou int. do PE
Nií intenzita donoru nií rozptyl akceptoru
55
Jak naloit se zkreslením?
PE ? PE-TxRed
donor
akceptor
CD10 PE-TxRed
vysoce exprimovaný znak do PE-TxRed
CD19 PE
56
Jak naloit se zkreslením?
PE ? PE-TxRed
donor
akceptor
CD8 PE-TxRed
- kvalitativní znak do PE-TxRed
CD3 PE
57
488nm Blue laser octagon
PerCP-Cy5.5 PerCP, PC5, Tricolor Cy-Chrome
PE-Dyomics647
PE
D
SSC
B
575/26
F
695/40
556 LP
655 LP
488/10
-
H
G
502 LP
735 LP
780/60
A
530/30
595 LP
610/20
PE-Cy7, PE-Alexa 750
E
C
FITC Alexa 488
PE-Texas Red ECD PE Dyomics590
58
633nm Red laser trigon
APC Alexa 633 Alexa 647 Dyomics 647
B
660/20
-
680 LP
720/40
A
Alexa 680
C
59
407nm Violet laser trigon
DAPI Hoechst Alexa 405 Pacific Blue
B
450/40
-
502 LP
530/30
A
Alexa Fluor 430
C