Fluorescenza - PowerPoint PPT Presentation
1 / 50
Title:
Fluorescenza
Description:
Un indicatore fluorescente un fluoroforo disegnato per essere ... e costruire chimere. mutazioni per farla adeguata alla misura di calcio negli organelli ... – PowerPoint PPT presentation
Number of Views:343
Avg rating:3.0/5.0
Title: Fluorescenza
1
Fluorescenza
2
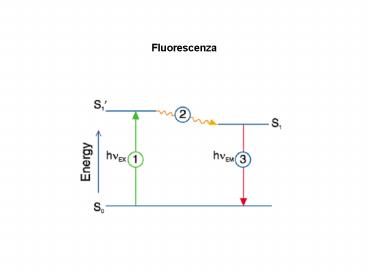
- La fluorescenza è il risultato di un processo a
tre stadi che avviene in alcune molecole
(idrocarburi poliaromatici o eterocicli) chiamati
fluorofori o coloranti fluorescenti. Un
indicatore fluorescente è un fluoroforo disegnato
per essere localizzato all'interno di una regione
specifica di uno specimen biologico o per
rispondere ad uno stimolo specifico. Il processo
risponsabile della fluorescenza dei composti
fluorescenti è illustrato mediante un semplice
diagramma di stato elettronico. - Stadio 1 eccitamento
- Un fotone di energia hv è fornito da una fonte
esterna (lampada a incandescenza, laser) e viene
assorbito dal fluoroforo creando uno stato
eccitato S'1. Questo processo differenza la
fluorescenza della chemioluminiscenza, in cui lo
stato eccitato è originato da una reazione
chimica. - Stadio 2 durata stato eccitato
- Lo stato eccitato perdura per un tempo definito
1x10-9 sec. Durante questo tempo il fluoroforo
subisce cambiamenti conformazionali ed è
sottoposto a molteplici possibili interazioni con
il suo intorno molecolare. Questi processi hanno
due consequenza importanti. 1) l'energia S1' è
dissipata parzialmente uno stato eccitato del
singoleto rilassato S1 dal quale origina
l'emissione fluorescente. 2) Non tutte le
molecole inizialmente eccitate dall'assorbimento
stato 1 ritornano allo stato basale mediante
emissione di fluorescenza. Altri processi
quenching collisionale, transferimento di energia
fluorescente ecc possono depopolare S1. Una
misura dell'estensione di questo processo è la
resa quantica di fluorescenza (fluorescence
quantum yield) che è il quoziente fra il numero
di fotoni emessi (stato 3) e il numero di fotoni
assorbiti. - Stato 3 emissione di fluorescenza
- Viene emesso un fotone di energia hvEM, e il
fluoroforo ritorna allo stato basale. Dovuto alla
dissipazione di energia durante il tempo nello
stato eccitato, l'energia di questo fotono è
minore, e pertanto la sua lunghezza d'onda è
maggiore di quella del fotono di eccitamento. La
differenza di energia o di lunghezza d'onda è
chiamata variazione di Stokes ed è fondamentale
per la sensibilità delle tecniche fluorescenti
perché permette il rivelamento dei fotoni emessi
contro un fondo basso, isolatidai fotoni di
eccitamento. Al cntrario, nella spettrofotometri
di assorbimento si richiede la misura della luce
trasmessa in relazione ad alti livelli di luche
incidente della stessa lunghezza d'onda.
3
(No Transcript)
4
Spettro di emissione e di eccitamento
5
Fluorescence Spectra
- The entire fluorescence process is cyclical.
Unless the fluorophore is irreversibly destroyed
in the excited state (an important phenomenon
known as photobleaching, see below), the same
fluorophore can be repeatedly excited and
detected. The fact that a single fluorophore can
generate many thousands of detectable photons is
fundamental to the high sensitivity of
fluorescence detection techniques. For polyatomic
molecules in solution, the discrete electronic
transitions represented by hEX and hEM in Figure
1 are replaced by rather broad energy spectra
called the fluorescence excitation spectrum and
fluorescence emission spectrum, respectively. The
bandwidths of these spectra are parameters of
particular importance for applications in which
two or more different fluorophores are
simultaneously detected (see below). With few
exceptions, the fluorescence excitation spectrum
of a single fluorophore species in dilute
solution is identical to its absorption spectrum.
Under the same conditions, the fluorescence
emission spectrum is independent of the
excitation wavelength, due to the partial
dissipation of excitation energy during the
excited-state lifetime, as illustrated in Figure
1. The emission intensity is proportional to the
amplitude of the fluorescence excitation spectrum
at the excitation wavelength (Figure 2). - http//probes.invitrogen.com/handbook/sections/000
1.html
6
Fluorescence Signals
- Fluorescence intensity is quantitatively
dependent on the same parameters as absorbance
defined by the BeerLambert law as the product of
the molar extinction coefficient, optical path
length and solute concentration as well as on
the fluorescence quantum yield of the dye and the
excitation source intensity and fluorescence
collection efficiency of the instrument. In
dilute solutions or suspensions, fluorescence
intensity is linearly proportional to these
parameters. When sample absorbance exceeds about
0.05 in a 1 cm pathlength, the relationship
becomes nonlinear and measurements may be
distorted by artifacts such as self-absorption
and the inner-filter effect. Because fluorescence
quantitation is dependent on the instrument,
fluorescent reference standards are essential for
calibrating measurements made at different times
or using different instrument configurations. To
meet these requirements, Molecular Probes offers
high-precision fluorescent microsphere reference
standards for fluorescence microscopy and flow
cytometry and a set of ready-made fluorescent
standard solutions for spectrofluorometry
7
- A spectrofluorometer is extremely flexible,
providing continuous ranges of excitation and
emission wavelengths. Laser-scanning microscopes
and flow cytometers, however, require probes that
are excitable at a single fixed wavelength. In
contemporary instruments, the excitation source
is usually the 488 nm spectral line of the
argon-ion laser. As shown in Figure 3, separation
of the fluorescence emission signal (S1) from
Rayleigh-scattered excitation light (EX) is
facilitated by a large fluorescence Stokes shift
(i.e., separation of A1 and E1). Biological
samples labeled with fluorescent probes typically
contain more than one fluorescent species, making
signal-isolation issues more complex. Additional
optical signals, represented in Figure 3 as S2,
may be due to background fluorescence or to a
second fluorescent probe.
8
Background Fluorescence
- Fluorescence detection sensitivity is severely
compromised by background signals, which may
originate from endogenous sample constituents
(referred to as autofluorescence) or from unbound
or nonspecifically bound probes (referred to as
reagent background). Detection of
autofluorescence can be minimized either by
selecting filters that reduce the transmission of
E2 relative to E1 or by selecting probes that
absorb and emit at longer wavelengths. Although
narrowing the fluorescence detection bandwidth
increases the resolution of E1 and E2, it also
compromises the overall fluorescence intensity
detected. Signal distortion caused by
autofluorescence of cells, tissues and biological
fluids is most readily minimized by using probes
that can be excited at gt500 nm. Furthermore, at
longer wavelengths, light scattering by dense
media such as tissues is much reduced, resulting
in greater penetration of the excitation light
9
Fluorescence detection of mixed species.
Excitation (EX) in overlapping absorption bands
A1 and A2 produces two fluorescent species with
spectra E1 and E2. Optical filters isolate
quantitative emission signals S1 and S2.
10
Fluorofori
Absorption and fluorescence spectral ranges for
28 fluorophores of current practical importance.
The range encompasses only those values of the
absorbance or the fluorescence emission that are
gt25 of the maximum value. Fluorophores are
arranged vertically in rank order of the maximum
molar extinction coefficient (max), in either
methanol or aqueous buffer as specified. Some
important excitation source lines are indicated
on the upper horizontal axis.
11
Fotobleaching
Comparison of photostability of green-fluorescent
antibody conjugates. The following fluorescent
goat antimouse IgG antibody conjugates were used
to detect mouse antihuman IgG antibody labeling
of human anti-nuclear antibodies in HEp-2 cells
on prefixed test slides (INOVA Diagnostics
Corp.) Oregon Green 514 (O6383, ), Alexa Fluor
488 (A11001, ), BODIPY FL (B2752, ), Oregon Green
488 (O6380, ) or fluorescein (F2761, ). Samples
were continuously illuminated and viewed on a
fluorescence microscope using a fluorescein
longpass filter set. Images were acquired every
five seconds. For each conjugate, three data
sets, representing different fields of view, were
averaged and then normalized to the same initial
fluorescence intensity value to facilitate
comparison.
12
Rilevamento della fluorescenza
- fonte di eccitamento
- fluoroforo
- filtri per isolare i fotoni di emissione da
quelli di eccitamento - rilevatore di fotoni di emissione con uscita che
permetta documentare i cambiamenti segnale
elettrico, immagini
13
Istrumenti
- spettrofluorimetro misura le proprietà medie dei
campioni (microL-mL) - microscopio a fluorescenza risolve la
fluorescenza in funzione di coordinate spaziali
in 2 o 3 dimensioni - citofluorimetro misura la fluorescenza per
cellula in una corrente di flusso, permettendo
l'identificazione e quantificazione di
sottopopolazioni in un campione
14
- Lintensità del segnale dipende da
- coefficiente di estinzione molare
- cammino ottico
- concentrazione del soluto
- resa quantica del colorante fluorescente
- intensità della fonte
- efficienza dell'istrumento collettore
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)
20
Misura della Ca con indicatori fluorescenti
- Indicatori fluorescenti che mostrano una
viariazione nello spettro dopo il legame di
calcio permettono lo studio delle variazioni
delle concentrazioni di calcio intracellulare
mediante la microscopia a fluorescenza, la
citofluorometria di flusso o la spettroscopia a
fluorescenza. Molti di questi indicatori sono
modificazioni dei chelanti non fluorescenti EGTA
e BAPTA - indicatori eccitati dal UV Fura-2, Indo-1 e
derivati - indicatori eccitati dalla luce visibile Fluo-3,
rhod-2 e derivati calcium green
21
(No Transcript)
22
Misure raziometriche
- In alcuni casi le forme libere e legate di un
indicatore ionico hanno spettri di emissione o de
assorbimento differenti. Il rapporto fra i
segnali può essere usato per monitorare la
costante di associazione e per calcolare la
concentrazioni ioniche. - Le misure raziometriche eliminano distorsioni
dovute a fotobleaching, variazioni nel
caricamento e ritenzione della sonda, e fattori
istrumentali (stabilità dellilluminazione).
23
(No Transcript)
24
(No Transcript)
25
(No Transcript)
26
(No Transcript)
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
- Figure 2. Simulated data demonstrating the
practical importance of ratiometric fluorescence
techniques. The figure represents an ion
indicator that exhibits a fluorescence intensity
increase in response to ion binding at wavelength
1 and a corresponding decrease at 3. Fluorescence
measured at an isosbestic point (2) is
independent of ion concentration. The
intracellular indicator concentration diminishes
rapidly due to photobleaching, leakage (assuming
the extracellular indicator is not detectable) or
some other process. The change of intracellular
ion concentration due to a stimulus applied at
the time indicated by the arrow is unambiguously
identified by recording the fluorescence
intensity ratios 1/3 or 1/2.
31
- Cai kd(R-Rmin)/(Rmax-R)(Sf,2/Sb,2)
- Kd è stata tabulata 224 nM
- I valori del Rapporto minimo e massimo vengono
determinati sperimentalmente nelle seguenti
condizioni - perfondendo il preparato con una soluzione
fisiologica priva di calcio aggiunto e contenente
EGTA e ionomicina - perfondendo con soluzione contenente calcio, in
presenza di ionomicina - Sf,2 Sonda libera F380- fondo
- Sb,2 Sonda satura F380-fondo
32
Aequorina e proteina fluorescente verde
istrumenti per lo studio dei segnali cellulari
- Omeostasi calcio
- traslocazione proteine
- espressione genica ecc
- possono essere indirizzate a localizzazioni
subcellulari
33
(A) The aequorin reaction, and (B) the Ca2
concentration response curve of recombinant
aequorin The fractional rate of aequorin
consumption is expressed as the ratio between the
emission of light at a defined Ca2 concentration
(L) and the maximal rate of light emission at a
saturating Ca2 concentration (Lmax).
34
Figure 2 Schematic representation of aequorin
chimaeras targeted to specific subcellular
locations The chimaeras represented localize to
the cytoplasm (cyt-AEQ), the mitochondrial matrix
(mt-AEQ), the mitochondrial intermembrane space
(mims-AEQ), the ER (er-AEQ), the sarcoplasmic
reticulum (sr-AEQ), the Golgi apparatus (go-AEQ),
the subplasmalemma region (pm-AEQ), the cytosol
or nucleus (depending on presence of
glucocorticoids cyt/nu-AEQ) or the nucleus only
(nu-AEQ). A white asterisk within the aequorin
portion denotes the D119A mutation. Abbreviations
are as follows COX 8, N-terminal fragment of
subunit VIII of cytochrome c oxidase GPD,
glycerol phosphate dehydrogenase L-VDJ-CH1,
domains of an Igg2b heavy chain CS,
calsequestrin ST-tm, sialyltransferase
transmembrane domain SNAP-25, synaptosomal-associ
ated protein of 25kDa NLS, nuclear localization
signal SB, steroid-binding domain.
35
- Aequorina deve essere microiniettata
- la reazione è irreversibile
- dopo il clonaggio è stato possibile esprimerla
senza microiniezione del peptide - e costruire chimere
- mutazioni per farla adeguata alla misura di
calcio negli organelli - inserimento di sequenze di localizzazione
36
Figure 3 Schematic representation of a
custom-built luminometer Cells expressing
functional aequorin probe are incubated in a
perfusion chamber, at 37C, in close proximity to
a photon-counting tube. The complete assemblage
is in the dark, to minimize extraneous signals.
The luminescence data are acquired by an
IBM-compatible computer via a photon-counting
board, and conversion of light signal into Ca2
concentration is carried out using an algorithm
based on the Ca2 response curve of aequorin.
Abbreviations amp/discr, amplifier/discriminator
pmt, photomultiplier tube.
37
Figure 4 Calibration of light data into Ca2
concentration values Shown are light emission (a)
and calculated values for the mitochondrial Ca2
concentration Ca2m (b) from a monolayer of
HeLa cells expressing mitochondrial aequorin.
Where indicated, the cells were challenged with
100µM histamine. At the end of the experiments
the cells were lysed with 100µM digitonin in a
hypotonic Ca2-rich solution (10mM CaCl2 in
water) to estimate the total photoprotein pool.
38
Measurement of Ca2 concentration using cytosolic
(a), mitochondrial (b) and ER (c) aequorin probes
39
Microscope set-up for ddetection of GFP protein A
digital imaging system, built on an
epifluorescence microscope, is equipped with
filter-wheels placed on the excitation and
emission light paths, a piezoelectric motor and a
CCD camera. The system is operated by software
that also permits analysis and computational
deblurring of the images. NA, numerical aperture
40
Time-course of cellular distribution of PKC
The transient expression of PKC-bIIHA1eGFP in
HeLa cells enables the pulse of this protein to
be tracked between the cytosol and the plasma
membrane, after the application of histamine
(100µM). The peaks at 7s and 47s are the clearest
ones after 1min PKC bII is again fully
cytosolic, and it can be recruited again by new
agonist stimulation (not shown). Time-lapse
movies are available at http//www.BiochemJ.org/b
j/355/bj3550001add.htm
41
(No Transcript)
42
(No Transcript)
43
FRETinterazione nello stato eccitato dipendente
dalla distanza in cui lemissione di un
fluoroforo è accoppiata alleccitamento di un
altro
Figure. Schematic representation of the FRET
spectral overlap integral.
44
Förster Radius The distance at which energy
transfer is 50 efficient (i.e., 50 of excited
donors are deactivated by FRET) is defined by the
Förster radius (Ro). The magnitude of Ro is
dependent on the spectral properties of the donor
and acceptor dyes (see Table)
45
Table. Typical Values of Ro. Donor Acceptor
Ro (Å) Fluorescein Tetramethylrhodamine 55
IAEDANS Fluorescein 46 EDANS Dabcyl
33 Fluorescein Fluorescein 44 BODIPY
FL BODIPY FL 57 Fluorescein QSY 7 and QSY
9 dyes 61
46
- Selected Applications of FRET
- Structure and conformation of proteins
- Spatial distribution and assembly of protein
complexes - Receptor/ligand interactions
- Immunoassays
- Probing interactions of single molecules
- Structure and conformation of nucleic acids
- Real-time PCR assays and SNP detection (Figure
8.112, Figure 8.113, Figure 8.114) - Detection of nucleic acid hybridization (Figure
8.110) - Primer-extension assays for detecting mutations
(Figure 8.113) - Automated DNA sequencing
- Distribution and transport of lipids
- Membrane fusion assays (Technical Focus
Lipid-Mixing Assays of Membrane Fusion) - Membrane potential sensing
- Fluorogenic protease substrates
- Indicators for cyclic AMP and zinc
47
Schematic representation of real-time PCR with
TaqMan primers. In the intact TaqMan probe,
energy is transferred (via FRET) from the
short-wavelength fluorophore on one end (green
circle) to the long-wavelength fluorophore on the
other end (red circle), quenching the
short-wavelength fluorescence. After
hybridization, the probe is susceptible to
degradation by the endonuclease activity of a
processing Taq polymerase. Upon degradation, FRET
is interrupted, increasing the fluorescence from
the short-wavelength fluorophore and decreasing
the fluorescence from the long-wavelength
fluorophore.
48
Eccimerisono dimeri nello stato attivato che
mostrano un spettro di emissione alterato
Principle Pyrene-labeled fatty acids (e.g., P31,
P96, P243 Section 13.2) can be biosynthetically
incorporated into viruses and cells in sufficient
quantities to produce the degree of labeling
required for long-wavelength pyrene excimer
fluorescence (Figure 13.8). This excimer
fluorescence is diminished upon fusion of labeled
membranes with unlabeled membranes (Figure 3).
Fusion can be monitored by following the increase
in the ratio of monomer (400 nm) to excimer
(470 nm) emission, with excitation at about 340
nm. This method appears to circumvent some of the
potential artifacts of the octadecyl rhodamine B
self-quenching technique and, therefore, provides
a useful alternative for viruscell fusion
applications.
Figure 3. Pictorial representation of a
lipid-mixing assay based on pyrene excimer
formation (Figure 13.8). Locally concentrated
pyrene-labeled lipid probes emit red-shifted
fluorescence due to formation of excimers
(excited state dimers). Probe dilution by
unlabeled lipids as a result of membrane fusion
is registered by the replacement of excimer
emission by blue-shifted monomer
fluorescence. Applications Applications of
pyrene excimer assays for membrane fusion are
described in footnoted references.
49
Self-quenching
Principle Lipid-mixing assays based on
self-quenching of octadecyl rhodamine were
originally described by Hoekstra and co-workers.
Octadecyl rhodamine B self-quenching occurs when
the probe is incorporated into membrane lipids at
concentrations of 110 mole percent. Unlike
phospholipid analogs, octadecyl rhodamine B can
readily be introduced into existing membranes in
large amounts. Fusion with unlabeled membranes
results in dilution of the probe, which is
accompanied by increasing fluorescence
(excitation/emission maxima 560/590 nm) . The
assay may be compromised by effects such as
spontaneous transfer of the probe to unlabeled
membranes, quenching of fluorescence by proteins
and probe-related inactivation of viruses the
prevalence of these effects is currently
debated FIGURE Pictorial representation of a
lipid-mixing assay based on fluorescence
self-quenching. Fluorescence of octadecyl
rhodamine B incorporated at gt1100 with respect
to host membrane lipids, is quenched due to
dyedye interactions. Fusion with unlabeled
membranes causes dispersion of the probe,
resulting in a fluorescence increase that is
represented here by a color change from black to
green. Applications The octadecyl rhodamine B
self-quenching assay is extensively used for
detecting viruscell fusion.
50
Trasferimento di energia studio di fusione di
membrane
Struck, Hoekstra and Pagano introduced
lipid-mixing assays based on NBDrhodamine energy
transfer. In this method (FIGURE 1), membranes
labeled with a combination of fluorescence energy
transfer donor and acceptor lipid probes
typically NBD-PE (N360, Section 13.2) and N-Rh-PE
(L1392, Section 13.2), respectively are mixed
with unlabeled membranes. Fluorescence resonance
energy transfer (FRET), detected as rhodamine
emission at 585 nm resulting from NBD excitation
at 470 nm, decreases when the average spatial
separation of the probes is increased upon fusion
of labeled membranes with unlabeled membranes.
The reverse detection scheme, in which FRET
increases upon fusion of membranes that have been
separately labeled with donor and acceptor
probes, has also proven to be a useful
lipid-mixing assay Figure . Pictorial
representation of a lipid-mixing assay based on
fluorescence resonance energy transfer (FRET).
The average spatial separation of the donor (D)
and acceptor (A) lipid probes increases upon
fusion of labeled membranes with unlabeled
membranes, resulting in decreased efficiency of
proximity-dependent FRET (represented by yellow
arrows). Decreased FRET efficiency is registered
by increased donor fluorescence intensity and
decreased acceptor fluorescence intensity..
Www.probes.com