Chapter 24' Coulometry - PowerPoint PPT Presentation
1 / 19
Title:
Chapter 24' Coulometry
Description:
We add supporting electrolyte to make analyte's migration nearly zero. ... supporting elyte: salt that migrates and carries current, and doesn't do redox ... – PowerPoint PPT presentation
Number of Views:1733
Avg rating:3.0/5.0
Title: Chapter 24' Coulometry
1
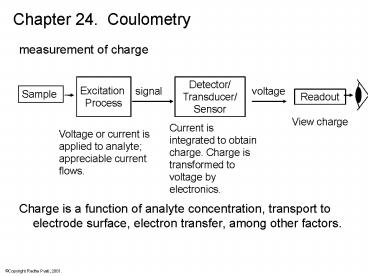
Chapter 24. Coulometry
- measurement of charge
- Charge is a function of analyte concentration,
transport to electrode surface, electron
transfer, among other factors.
Detector/ Transducer/ Sensor
Excitation Process
voltage
signal
Sample
Readout
View charge
Current is integrated to obtain charge. Charge is
transformed to voltage by electronics.
Voltage or current is applied to analyte
appreciable current flows.
2
- constant potential (potentiostatic)
- hold potential constant, measure current,
integrate over time to get charge. - constant current (galvanostatic or amperostatic)
- hold current constant, electrolytically generate
a reactant - this reactant is the titrant in a coulometric
titration. - titrant reacts with analyte until analyte
consumed. Measure length of time that current
flowed. - electrogravimetry - measurement of mass of solid
product deposited on one electrode - Q it Q charge (C) i current (A C/s)
- t time (s)
3
Chapter 25. Voltammetry
I. Concept
- Current is a function of
- analyte concentration
- how fast analyte moves to electrode surface
- rate of electron transfer to sample
- voltage, time...
4
II. Excitation process
- A. What happens when a voltage is applied to an
electrode in solution containing a redox species? - generic redox species O
- O e- --gt R E -0.500 V v. SCE
- Imagine that we have a Pt etrode in soln at an
initial potential of 0.000 V v. SCE and we switch
potential to -0.700 V. - First
supporting electrolyte
O redox
solvent
5
B. Events that happen
- 1. supporting electrolyte forms an electrical
double layer
cation movement to etrode causes an initial spike
in current Formation of double layer is good
because it ensures that no electric field exists
across whole soln (requires 1001 conc ratio of
supporting elyteredox species).
6
2. Electron transfer rxn
O is converted to R at etrode surface.
?R
Eapp -0.7
?R
A depletion region of O develops - a region in
which conc of O is zero.
- How does more O get to etrode surface?
- mass transport mechanisms
7
C. Mass transport to the etrode
- 1. Migration - movement in response to electric
field. We add supporting electrolyte to make
analytes migration nearly zero. (fraction of
current carried by analyte ? zero) - 2. Convection
- stirring
- rotated disk etrode (RDE)
- Levich equation
- A etrode area
- w angular velocity (s-1) 2p rotation
speed - DO diffusion coefficient of O (cm2/s)
- n kinematic viscosity (cm2/s)
- CO bulk conc (mol/cm3) - (not surface conc)
8
2. Convection (contd)
- concentration profile in solution - x distance
from etrode surface
1
conc at distance x relative to bulk conc
0
dimensionless distance from etrode surface
At what distance does depletion region end and
bulk region begin when D 1 x 10-5 cm2/s and
etrode spins at 31 rpm?
9
2. Convection (contd)
- RRDE (rotated ring-disk etrode) - permits study
of rxn mechanisms
disk O ne --gt R
10
3. Diffusion
- In experiments relying upon diffusion, no
convection is desired, soln is quiescent. - Consider an etrode at which we step voltage
beyond E of redox couple. - Concentration profile in soln
1
x distance from etrode surface
0
11
3. Diffusion (contd)
- Very common technique cyclic voltammetry -
voltage is varied linearly as a function of time. - Conc profile hard to draw because both potential
and current are varying with time.
Randles-Sevcik equation v scan rate
(V/s) Polarography - older technique using
dropping Hg etrode
12
4. Microelectrodes
- Diffusion
- Linear (planar) vs. radial (spherical)
- microelectrodes rely on radial diffusion
- advantages include
- steady-state measurement (not dependent on
time) - less iR because less current
- less capacitance
- for a microdisk, iL 4nFrDOCO r etrode
radius (cm)
etrode
13
D. Voltage programs
- We can apply
- a single dc voltage for a desired period of time
(chronoamperometry) - a voltage that varies linearly with time (linear
sweep voltammetry or cyclic voltammetry) - a voltage that varies linearly with time but also
has regular pulses to increase sensitivity or
remove background signal (differential pulse
voltammetry (DPV) and square wave voltammetry) - a voltage that collects metals and then oxidizes
them (anodic stripping voltammetry - ASV)
14
D. Voltage programs revisited
- 1. DPV and square wave voltammetry
- 2. ASV
- metal cations reduced to metal, which dissolves
in Hg drop - Mn ne- --gt M(Hg)
- metal solid is then oxidized - highly
concentrated in Hg - M(Hg) --gt Mn ne-
- (concentrated)
- sensitive technique
15
E. Solutions and electrodes
- 1. Solutions redox couple solvent
supporting electrolyte - supporting elyte salt that migrates and
carries current, and doesnt do redox in your
potential window of interest - a wide potential window is desirable
- water - good for oxidations, not reductions
except on Hg supporting elytes lots of salts - nonaqueous solvents acetonitrile,
dimethylformamide, etc. - supporting electrolytes tetraalkylammonium BF4,
PF6, ClO4 - Oxygen is fairly easily reduced - we remove it by
deoxygenating with an inert gas (N2, Ar).
16
2. Electrodes
- working etrode (WE) is where redox activity
occurs - auxiliary etrode (AE) catches current flow from
WE - reference etrode (RE) establishes potential of WE
- a. working etrode materials
- Pt, Au, C, semiconductors
- Hg - messy but good for reductions in water.
Not good for oxidations. - b. auxiliary etrodes similar materials, large
in area - c. reference etrodes real vs. quasi -
- real refs have an actual redox couple (e.g.
Ag/AgCl) - quasi refs (QRE) - a wire at which some (unknown)
redox process occurs in soln. QREs OK if
currents are needed but not potentials.
17
III. Detection
- Current is measured and converted to voltage for
readout. - (gain or sensitivity is set using electronics,
e.g. 10 mA/V) - To minimize iR drop - three etrode potentiostat
18
IV. Advantages and disadvantages
- Advantages
- sensitive (good for quantitation)
- instrumentation is relatively inexpensive
- Disadvantages
- not so good at identification
- not too selective (although this is changing)
19
V. Some Applications
- A. Elucidation of rxn mechanism, or stability of
a cmpd - EC
- CE
- B. Chemically modified electrodes
- putting a layer on the electrode surface that
only allows attachment of analyte of interest - doing electrochemistry on attached analyte
- C. ECL (electrochemiluminescence)