Compton Effect - PowerPoint PPT Presentation
Title:
Compton Effect
Description:
Compton Effect. 1923 Compton performed an experiment which supported this idea. directed a beam of x-rays of wavelength onto a carbon target ... – PowerPoint PPT presentation
Number of Views:2196
Avg rating:3.0/5.0
Title: Compton Effect
1
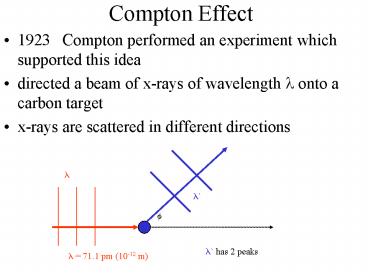
Compton Effect
- 1923 Compton performed an experiment which
supported this idea - directed a beam of x-rays of wavelength ? onto a
carbon target - x-rays are scattered in different directions
? has 2 peaks
? 71.1 pm (10-12 m)
2
(No Transcript)
3
Compton Scattering
- Wavelength ? of scattered x-rays has two peaks
- these occur at ? and ? ??
- ?? gt0 is the Compton shift
- classical physics predicts ?? 0
- Quantum picture
- a single photon interacts with electrons in the
target - light behaves like a particle of energy
Ehfhc/? and momentum ph/ ? gt a collision
4
Compton Scattering
- Conservation of energy E E K
- gt E lt E gt f lt f gt ? gt ?
- X-ray momentum ph/? p h/?
- electron momentum pe ?mev
5
Compton Scattering
- Conservation of energy E E K
- gt E lt E gt f lt f gt ? gt ?
- X-ray momentum ph/? p h/?
- electron momentum pe ?mev
6
X-ray scattering
- Energy and momentum are conserved
- Momentum is a vector! Fdp/dt0 gt p constant
7
(No Transcript)
8
X-ray Scattering
- 3 equations in 5 variables ?, ?,v,?,?
- eliminate the electron variables v, ? gt find
?(v) - ? - ? (h/mec) (1 - cos?)
- ?c (1 - cos?) ?c is Compton
wavelength of the electron
9
Compton Scattering
- ? - ? ? c (1 - cos?)
- ?0 gt ? ?
- ??/2 gt ? ? ?c
- ? ? gt ? ? 2 ?c
- why are there two peaks?
10
Compton Scattering
- loosely bound electrons in Carbon are ejected
and the x-rays are scattered - ? - ? (h/mec) (1 - cos?)
- tightly bound electrons are not ejected
gt photon interacts with entire carbon atom - mass 22,000 me gt ?? reduced by this
factor
11
Problem
- An x-ray beam of wavelength 0.01 nm strikes a
target containing free electrons. Consider the
xrays scattered back at 1800 - Determine (a) change in wavelength of the xrays
(b) change in photon energy between incident and
scattered beams (c) the kinetic energy
transferred to the electron (d) the electrons
direction of motion
12
Solution
- X-ray beam has ?.01 nm 10 pm
- ?1800
- ? - ? (h/mec) (1 - cos?)
- ?c (1 - cos?) ?c is Compton
wavelength of the electron - (a) ??(h/cme)(1-cos(180)) 2h/cme 2(6.63x10-3
4)/(3x108)(9.11x10-31) 2(2.43 pm) 4.86 pm - (b) ?E hc/ ? -hc/?
(6.63x10-34)(3x108)1/14.86 -1/10/(10-12)
-.65x10-14 J -.41x105 eV -41 keV
13
Solution
- (c) K (electron) 41 keV
- (d) direction of electron?
- Momentum conserved gt electron moves forward
?p
14
Photons Revealed
- Can we devise an experiment where both the wave
and photon characteristics of light are involved?
- Photon has 50 chance of being transmitted or
reflected at B - reduce light beam energy to that of a single
photon - if photon picture is correct we get
anticoincidences - experiments were not convincing
15
Designated Photons
- 1986 Grangier, Roger, Aspect replaced source S by
beam of calcium ions
- Calcium excited by a laser and emits two photons
- trigger photon turns detectors on and off
(emitted and absorbed!) - designated photon demonstrated anticoincidences!
- Supported the photon picture! - no wave
interpretation possible - other modifications are possible to demonstrate
both wave and photon properties of light - see
section 45-6
16
Slowing Atoms by Photon Bombardment
- A gas of atoms at room temperature is in constant
motion - for argon gas at T3000K, vrms (3kT/m)1/2
430 m/s ! - How can we use photons to slow these atoms down?
- Consider that the atom absorbs a photon with ph/?
Typically a change of few cm/s due to a single
photon
- Atoms moving in the same direction as the laser
are speeded up gt no net slowing down
17
Laser Cooling
- When a photon is absorbed, the atom moves from
the ground state to an excited state - excited state does not have a well defined energy
since it only exists for a short time gt
uncertainty principle
Probability of absorption by atom at rest
Atom illuminated by two beams
?laser greater than peak value
Photons absorbed from L slow it down and those
absorbed from R speed it up gt do these effects
cancel?
18
Laser Cooling
- Doppler effect
- atom detects L as a higher f or lower ?L lt ?laser
- atom detects R as a lower f or higher ?R gt ?laser
- probabilities are not the same!
- net reduction in speed results
- experiments use 6 laser beams
trap