Physicochemical Properties in Relation to Biological Action - PowerPoint PPT Presentation
1 / 38
Title:
Physicochemical Properties in Relation to Biological Action
Description:
Bioavailability is related to Absorption. Fraction of drug that ... You're the Pharmacist... Cetirizine (Zyrtec) Clemastine (Travist) Olopatadine (Patanol) ... – PowerPoint PPT presentation
Number of Views:5480
Avg rating:3.0/5.0
Title: Physicochemical Properties in Relation to Biological Action
1
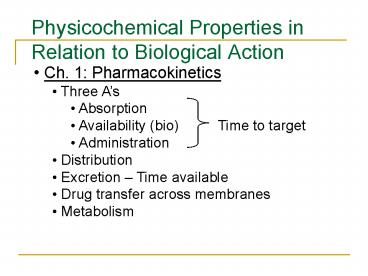
Physicochemical Properties in Relation to
Biological Action
- Ch. 1 Pharmacokinetics
- Three As
- Absorption
- Availability (bio) Time to target
- Administration
- Distribution
- Excretion Time available
- Drug transfer across membranes
- Metabolism
2
Drug Distribution
3
Bioavailability
- Bioavailability is related to Absorption
- Fraction of drug that gets to its target
- Routes
- Oral Ingestion (P.O.)
- Sublingual
- Rectal
- Parenteral
- Intravenous (I.V.)
- Subcutaneous (S.C.)
- Intramuscular (I.M.)
- Intraarterial (I.A.)
- Intrathecal (I.T.)
- Pulmonary
- Topical
- Mucous Membranes
- Skin
- Eye
4
Drug Distribution
- Follows Administration ? Absorption
- Interstitial Intercellular fluids
- Rate of delivery and distribution
- Cardiac output
- Regional blood flow
- Tissue volume
- Phase I
- Liver, kidney, brain get most (well-perfused)
- Muscle, viscera, fat ? SLOWER
- Phase II
- Minutes ? hours before tissue at equilibrium with
blood - Determined by partitioning (more mass in Phase
II) - Factors
- Lipid solubility
- Binding to plasma proteins
5
Distribution cont
- Plasma Proteins
- In bloodstream drug ? protein (Albumin)
- Affinity Availability ? Rate of delivery
- Tissues
- As much as 1000x
- Affinity Receptor types
- Redistribution
- Termination ? metabolism excretion
- Ex Lipid soluble anesthetics
- High blood flow to the brain ? high brain
concentration FAST! - CNS CSF
- Dependent on ability to cross tight junctions
(BBB) - Requires I.T. injection of many agents
- Placental Transfer
- Factors Lipid solubility, plasma binding, degree
of ionization
6
Excretion
- Drugs eliminated
- Unchanged
- As metabolites
- Elimination more efficient for polar molecules
- Lipid soluble ? more polar ? excretion
- Renal decrease 1 per year in adulthood!
- Glomerular filtration (MW lt 40,000)
- Tubular secretion
- Passive tubular reabsorption
- Biliary Fecal
- Enterohepatic recycling ? prolong drug effects
- Other Routes
- Sweat, saliva, tears, skin, hair, breast milk,
exhalation - Not significant! (Forensic utility!)
7
Common Administration Routes
Absorption Pattern
Limitation Precautions
Special Utility
Route
8
Drug Distribution
9
Cell Membranes
- Lipid bilayer noncovalent
- Phospho-, glycolipids, cholesterol
- Most water permeable
- Passive diffusion most drugs
- Osmotic/hydrostatic pressure
- Embedded Proteins Drug targets!
- Receptors external stimuli
- Ion channels gates
- Transporters active transport
- Signaling pathways
10
Membranes Fluid Mosaic Model
11
Fluid Mosaic Model - 4 Mechanisms
- Passive diffusion
- Most drugs!
- Function of the lipid solubility of the drug
(partition coefficient) - Drug moves from highest to lowest conc.
- Unionized species of the drug only!
- This is a first order process!
- Rate Drug
12
Fluid Mosaic Model cont
- Carrier Mediated or Active Absorption
- Few drugs and nutrients absorbed this way
- Structural similarities between drug and natural
substrate - Differences with passive transport
- Transport is against the concentration gradient
- Saturable at high drug concentrations-limited
number of carriers to shuttle the drug across the
membrane - Molecular specificity leads to competition
between drug and natural substrate ? decreased
drug transport - Requires energy
- Highest affinity transported more rapidly
13
Fluid Mosaic Model cont
- Convective absorption
- Small molecules (lt 4A radius)
- Travel through pores - sieving effect
- Saturable
- Ion pair absorption
- Equivalent to phase transfer that occurs with
quaternary ammonium cations and an anion
14
Acid/Base Properties
- Human body is 70-75 water
- 55 L per 160 lb (55 kg) individual
- Average drug molecule 200 g/mole
- 20 mg of drug would yield a 2 mM solution
- Dealing with DILUTE solutions
- Brönsted Lowry best describes this
- Acid H donor, Base H acceptor
- Drugs acids, bases, or both (Amphoteric)
- Physiologic pH 7.4
15
Daily Wisdom
16
Conjugate Acid - Base
- For an acid (ex. R-COOH)
- HA H A-
- For a base (ex. R-NH2)
- BH H B
Conjugate Acid Conjugate base
R-NH3 R-NH2
17
Conjugate Acid - Base
Dopamine - Neurotransmitter
- Questions
- Conjugate acid or base?
- Where is the chemical form found?
- Where is the drug best absorbed? Why?
- Conjugate Acid
- Stomach
- Conjugate Base
- Small Intestine
3. Basic drugs are best absorbed in the Small
Intestine!
RATE!!!
18
Conjugate Acid/Base Pairs
19
pH Effects on Absorption
20
Drug Transfer Acidic Drugs
21
Drug Transfer Acidic Drugs
22
Drug Transfer Basic Drugs
23
Drug Transfer Basic Drugs
24
Ionization vs. pH
- Henderson-Hasselbach equationpH pKa log
(conjugate base/acid) - Example What is the ratio of pseudoephedrine to
pseudoephedrine HCl (pKa 9.9) in the small
intestine at pH 8.0?8.0 9.9 log
(pseudoephedrine/HCl salt)log
(pseudoephedrine/HCl salt) -9.9 8.0
-1.9 (pseudoephedrine/HCl salt) 0.0126 - 13 pseudoephedrine per 1000 pseudoephedrine HCl
25
Ionized vs. pH
- For HA acids
- ionization 100/(1 10(pKa pH))
- For BH acids ionization 100/(1 10(pH
pKa))
Example Percentage ionized pseudoephedrine HCl
(pKa 9.9) in the small intestine at pH
8.0? ionization 100/(1 10(8.0 9.9))
ionization 100/(1 0.0126) ionization
100/1.0126 ionization 98.76
26
pH Effects on Absorption
27
Lipid Solubility Partition Coefficient
- n-octanol / water partition coefficient
- Drug n-octanol / Drug water
28
Factors that determine solubility
- Dipole Moments Ex Dichlorobenzene
- A dipole moment (m Debyes) results from the
UNEQUAL sharing of electrons two atoms.
29
Dipoles cont
- In general molecules that possess permanent
dipole moments are called polar molecules - The overall polarity of a molecule is the
summation of the all the dipole vectors within a
molecule. - It is rare in a complex molecule like a drug that
a plane of symmetry exists such that all dipoles
effectively cancel each other out.
30
Ans. (a) gt (c) gt (b) (d)
31
Polarizability
- Induced Polarity Polarizability
- Adjacent molecules induce a functional group in
a molecule to develop a dipole. - Ex Distortion of Benzene p-electron cloud by
interaction with a DMF molecule. - Total Polarity Polarizability Dipole moment
32
Other influences on polarity
- Intermolecular Forces
- Dipole Dipole interactions
- Dipole Induced Dipole interactions
- Induced Induced dipole interactions (London
Forces) - Ion Dipole dipole interactions
- Ion Induced Dipole interactions
- Hydrogen bonds - 2 to 8 kcal/mole
- 1 - 3 collectively as Van derWaals forces and
4-5 afford 1-10 kcal/mole
33
Hydrogen Bonding
34
Hydrogen Bonding
- Affect physical chemical drug properties
- Determine strength of drug protein interaction
- Influences bioavailability
- Acidic drug Albumin
- Basic drug a1-acid glycoprotein
- Adsorption vs. Absorption
35
Parameters to predict activity
- Hammetts s constant Useful for determining the
effect of meta and para substituents in aromatic
compounds a measure of resonance versus
inductive effects - Tafts steric parameters - Es
- Chartons steric parameters v
- Verloops multidimensional steric parameters
- Molar refractivity MR
- One of the most popular methods
- Involves a molecules refractive index, molecular
weight and density - Essentially a measure of a molecules bulk and
electronic character
36
Problems
- QSAR or Quantitative Structure Activity
Relationships - Only a predictive tool
- Often inaccurate
- Methods often do NOT directly predict
biological/pharmacological activity - Structural parameters only!
- Computer graphics allow 3-D structural predictions
37
(No Transcript)
38
Youre the Pharmacist
Cetirizine (Zyrtec)
Olopatadine (Patanol)