Hesss Law Problems - PowerPoint PPT Presentation
1 / 17
Title:
Hesss Law Problems
Description:
1. Pick reactions that have reacts and prods already in place (know don't know) 2. ... 1. Pick reactions that have reacts and prods already in place (know don't know) ... – PowerPoint PPT presentation
Number of Views:190
Avg rating:3.0/5.0
Title: Hesss Law Problems
1
Hesss Law Problems
- How to go about looking at these types of
problems.
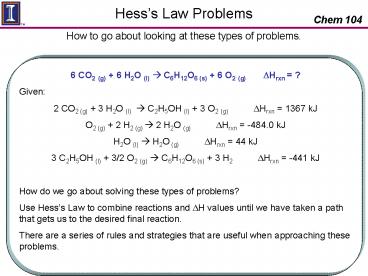
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 2 CO2 (g) 3 H2O (l) ?
C2H5OH (l) 3 O2 (g) DHrxn 1367 kJ O2
(g) 2 H2 (g) ? 2 H2O (g) DHrxn
-484.0 kJ H2O (l) ? H2O (g) DHrxn 44
kJ 3 C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ How do we go about
solving these types of problems? Use Hesss Law
to combine reactions and DH values until we have
taken a path that gets us to the desired final
reaction. There are a series of rules and
strategies that are useful when approaching these
problems.
2
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 2 CO2 (g) 3 H2O (l) ?
C2H5OH (l) 3 O2 (g) DHrxn 1367 kJ O2
(g) 2 H2 (g) ? 2 H2O (g) DHrxn
-484.0 kJ H2O (l) ? H2O (g) DHrxn 44
kJ 3 C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ Strategy1. Pick
reactions that have reacts and prods already in
place (know ? dont know)2.3.4. Rules1.2.3.
3
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 2 CO2 (g) 3 H2O (l) ?
C2H5OH (l) 3 O2 (g) DHrxn 1367 kJ 3
C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ Strategy1. Pick reactions
that have reacts and prods already in place (know
? dont know)2. Set up reactions to cancel out
compounds you dont need in final
reaction3.4. Rules1.2.3.
x 3
4
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ? 3
C2H5OH (l) 9 O2 (g) DHrxn 1367 kJ 3
C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ Remember, DH is extensive, so
if we multiply the reaction by 3, what MUST we do
to DH? Strategy1. Pick reactions that have
reacts and prods already in place (know ? dont
know)2. Set up reactions to cancel out
compounds you dont need in final
reaction3.4. Rules1. 2.3.
5
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ? 3
C2H5OH (l) 9 O2 (g) DHrxn 1367 kJ 3
C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ Remember, DH is extensive, so
if we multiply the reaction by 3, what MUST we do
to DH? Strategy1. Pick reactions that have
reacts and prods already in place (know ? dont
know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
4. Rules1. Multiply reaction by 3? Multiply
DH by 3 2.3.
6
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ? 3
C2H5OH (l) 9 O2 (g) DHrxn 4101 kJ 3
C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ Remember, DH is extensive, so
if we multiply the reaction by 3, what MUST we do
to DH? Strategy1. Pick reactions that have
reacts and prods already in place (know ? dont
know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
4. Rules1. Multiply reaction by 3? Multiply
DH by 3 2.3.
7
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ? 3
C2H5OH (l) 9 O2 (g) DHrxn 4101 kJ 3
C2H5OH (l) 3/2 O2 (g) ? C6H12O6 (s) 3 H2
DHrxn -441 kJ 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 3 H2 (g) 15/2 O2 (g)
DHrxn 3660 kJ Strategy1. Pick reactions
that have reacts and prods already in place (know
? dont know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Rules1.
Multiply reaction by 3? Multiply DH by 3 2.3.
15/2
8
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 3 H2 (g) 15/2 O2 (g)
DHrxn 3660 kJ O2 (g) 2 H2 (g) ? 2 H2O (g)
DHrxn -484.0 kJ H2O (l) ? H2O (g)
DHrxn 44 kJ Strategy1. Pick reactions that
have reacts and prods already in place (know ?
dont know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Take the
combined reaction and Lather, Rinse, and Repeat
as neccessary Rules1. Multiply reaction by 3?
Multiply DH by 3 2.3.
9
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 3 H2 (g) 15/2 O2 (g)
DHrxn 3660 kJ O2 (g) 2 H2 (g) ? 2 H2O (g)
DHrxn -484.0 kJ Strategy1. Pick
reactions that have reacts and prods already in
place (know ? dont know)2. Set up reactions to
cancel out compounds you dont need in final
reaction3. Cancel and combine reactions 4.
Take the combined reaction and Lather, Rinse, and
Repeat as neccessary Rules1. Multiply reaction
by 3? Multiply DH by 3 2.3.
X 3/2
10
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 3 H2 (g) 15/2 O2 (g)
DHrxn 3660 kJ 3/2 O2 (g) 3 H2 (g) ? 3 H2O (g)
DHrxn -726.0 kJ Can we cancel out the
water??? Strategy1. Pick reactions that have
reacts and prods already in place (know ? dont
know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Take the
combined reaction and Lather, Rinse, and Repeat
as neccessary Rules1. Multiply reaction by 3?
Multiply DH by 3 2.3.
12/2 6
11
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 3 H2 (g) 15/2 O2 (g)
DHrxn 3660 kJ 3/2 O2 (g) 3 H2 (g) ? 3 H2O (g)
DHrxn -726.0 kJ 6 CO2 (g) 9 H2O (l)
? C6H12O6 (s) 6 O2 (g) 3 H2O (g)
DHrxn 2934 kJ Strategy1. Pick reactions that
have reacts and prods already in place (know ?
dont know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Take the
combined reaction and Lather, Rinse, and Repeat
as neccessary Rules1. Multiply reaction by 3?
Multiply DH by 3 2. States of matter MUST be
the same to cancel out3.
12/2 6
12
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ H2O (l) ? H2O (g) DHrxn 44
kJ Need to reverse the reaction Strategy1.
Pick reactions that have reacts and prods already
in place (know ? dont know)2. Set up reactions
to cancel out compounds you dont need in final
reaction3. Cancel and combine reactions 4.
Take the combined reaction and Lather, Rinse, and
Repeat as neccessary Rules1. Multiply reaction
by 3? Multiply DH by 3 2. States of matter
MUST be the same to cancel out3.
13
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ H2O (g) ? H2O (l) DHrxn 44
kJ Reaction has been reversed, so that changes
the direction of the heat flow Strategy1.
Pick reactions that have reacts and prods already
in place (know ? dont know)2. Set up reactions
to cancel out compounds you dont need in final
reaction3. Cancel and combine reactions 4.
Take the combined reaction and Lather, Rinse, and
Repeat as neccessary Rules1. Multiply reaction
by 3? Multiply DH by 3 2. States of matter
must be the same to cancel out3.
14
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ H2O (g) ? H2O (l) DHrxn 44
kJ Reaction has been reversed, so that changes
the direction of the heat flow Strategy1.
Pick reactions that have reacts and prods already
in place (know ? dont know)2. Set up reactions
to cancel out compounds you dont need in final
reaction3. Cancel and combine reactions 4.
Take the combined reaction and Lather, Rinse, and
Repeat as neccessary Rules1. Multiply reaction
by 3? Multiply DH by 3 2. States of matter
must be the same to cancel out3. If rxn is
reversed, change the sign of DH
15
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ H2O (g) ? H2O (l) DHrxn -44
kJ Strategy1. Pick reactions that have
reacts and prods already in place (know ? dont
know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Take the
combined reaction and Lather, Rinse, and Repeat
as neccessary Rules1. Multiply reaction by 3?
Multiply DH by 3 2. States of matter must be
the same to cancel out3. If rxn is reversed,
change the sign of DH
16
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ H2O (g) ? H2O (l) DHrxn -44
kJ Multiply by 3 Strategy1. Pick reactions
that have reacts and prods already in place (know
? dont know)2. Set up reactions to cancel out
compounds you dont need in final reaction3.
Cancel and combine reactions 4. Take the
combined reaction and Lather, Rinse, and Repeat
as neccessary Rules1. Multiply reaction by 3?
Multiply DH by 3 2. States of matter must be
the same to cancel out3. If rxn is reversed,
change the sign of DH
17
Hesss Law Problems
- How to go about looking at these types of
problems.
6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6 O2 (g)
DHrxn ? Given 6 CO2 (g) 9 H2O (l) ?
C6H12O6 (s) 6 O2 (g) 3 H2O (g) DHrxn
2934 kJ 3 H2O (g) ? 3 H2O (l) DHrxn
-132 kJ 6 CO2 (g) 6 H2O (l) ? C6H12O6 (s) 6
O2 (g) DHrxn 2802 kJ Strategy1.
Pick reactions that have reacts and prods already
in place (know ? dont know)2. Set up reactions
to cancel out compounds you dont need in final
reaction3. Cancel and combine reactions 4.
Take the combined reaction and Lather, Rinse, and
Repeat as neccessary Rules1. Multiply reaction
by 3? Multiply DH by 3 2. States of matter
must be the same to cancel out3. If rxn is
reversed, change the sign of DH
6