ATLAS: Efficacy at weeks 12 and 24 - PowerPoint PPT Presentation
Title:
ATLAS: Efficacy at weeks 12 and 24
Description:
ATLAS: Efficacy at weeks 12 and 24 Placebo (n=107), % Adalimumab 40 mg (n=208), % Measure ... Conceptis Technologies Other titles: – PowerPoint PPT presentation
Number of Views:84
Avg rating:3.0/5.0
Title: ATLAS: Efficacy at weeks 12 and 24
1
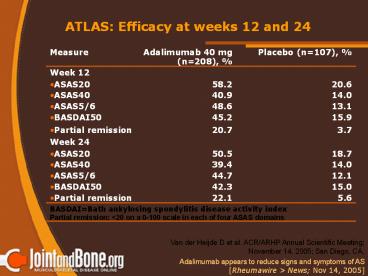
ATLAS Efficacy at weeks 12 and 24
Measure Adalimumab 40 mg (n208), Placebo (n107),
Week 12
ASAS20 58.2 20.6
ASAS40 40.9 14.0
ASAS5/6 48.6 13.1
BASDAI50 45.2 15.9
Partial remission 20.7 3.7
Week 24
ASAS20 50.5 18.7
ASAS40 39.4 14.0
ASAS5/6 44.7 12.1
BASDAI50 42.3 15.0
Partial remission 22.1 5.6
BASDAIBath ankylosing spondylitis disease
activity index Partial remission lt20 on a 0-100
scale in each of four ASAS domains
Van der Heijde D et al. ACR/ARHP Annual
Scientific Meeting November 14, 2005 San
Diego, CA.
Adalimumab appears to reduce signs and symptoms
of AS Rheumawire gt News Nov 14, 2005
2
Adverse events (occurring gt5 through week 24)
Event Adalimumab 40 mg (n208), Placebo, (n107),
Nasopharyngitis 26 (12.5) 8 (7.5)
Injection-site reaction 22 (10.6) 3 (2.8)
Headache 20 (9.6) 9 (8.4)
Van der Heijde D et al. ACR/ARHP Annual
Scientific Meeting November 14, 2005 San
Diego, CA.
Adalimumab appears to reduce signs and symptoms
of AS Rheumawire gt News Nov 15, 2005