Pure Substances - PowerPoint PPT Presentation
1 / 10
Title:
Pure Substances
Description:
Pure Substances Cannot be broken down into simpler substances and still have the same properties. Pure Substances Elements The purest substances known. – PowerPoint PPT presentation
Number of Views:261
Avg rating:3.0/5.0
Title: Pure Substances
1
(No Transcript)
2
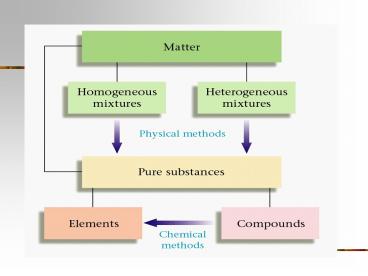
Pure Substances
- Cannot be broken down into simpler substances and
still have the same properties.
3
Pure Substances
- Elements
- The purest substances known.
- They contain all the same type of atom.
4
Pure Substances
- Compounds
- Combinations of atoms that are bonded together.
http//www.cybered.net/library/Teaching_Resources
/Chemistry/Naming_Chemical_Compounds/Image_Gallery
/NamingChem-Compounds.jpg
5
Mixtures
- Combinations of different types of matter that
can easily be separated from one another.
6
Mixtures
- Homogeneous Mixtures
- Look the same throughout.
- Include solutions, colloids, and suspensions.
http//www.guided-wave.com/_img/userfiles/Image/C
hemicalFlasks.jpg
7
Solutions have one substance that is so small it
isdissolved in another substance
8
Colloids and the Tyndall Effect Particles are
larger in a colloid than in a solution
http//cwx.prenhall.com/petrucci/medialib/media_p
ortfolio/text_images/FG14_21.JPG
9
A suspension has particles that eventually will
settle to the bottom.
http//cwx.prenhall.com/petrucci/medialib/media_p
ortfolio/text_images/FG14_09.JPG
10
Mixtures
- Heterogeneous Mixtures
- These have two or more visible parts.
http//www.chemistry.org/portal/a/c/s/1/resources
/ACS/ACSContent/wondernet/images/wn_float_oil.jpg