Topic 2 - PowerPoint PPT Presentation
1 / 24
Title:
Topic 2
Description:
Monosaccharide Drawing Practice. Look up the molecular structures of the three types of monosaccharides: alpha-glucose. beta-glucose. ribose. And perform the following: – PowerPoint PPT presentation
Number of Views:67
Avg rating:3.0/5.0
Title: Topic 2
1
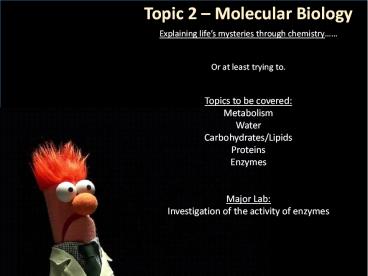
Topic 2 Molecular Biology
Explaining lifes mysteries through
chemistry Or at least trying to. Topics to
be covered Metabolism Water Carbohydrates/Lipids
Proteins Enzymes Major Lab Investigation of
the activity of enzymes
2
Molecular Biology came about because of the need
for humans to distinguish living material from
non-living material So naturally, they set
out to make urine in a lab.
Vitalism the belief that living organisms and
non-living organisms have different properties
- Called the vitalistic element, this was
believed to distinguish living from non-living
matter - This theory was disproved by a chemist
Friedrich Woehler. - He synthesized artificial urea by mixing cyanic
acid and ammonium. - This discovery was the first time an organic
substance was made from inorganic material
3
(No Transcript)
4
The Periodic Table of Elements! Parts we care
about are highlighted in green. These elements
are often used in the construction of Organic
Compounds
Organic compounds are found in living organisms
and contain carbon. 15 Minute Inquiry Research
one of the elements found in living systems and
determine the following -Structure (Draw it)
-Number of bonds it can form -Uses in living
systems -Example of a compound found in the
human body containing your element. Compile your
information on a whiteboard and turn in once
done.
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
Carbon as the basis of all life Why?
How many bonds can it form with other
molecules? What types of bonds is it going to
form most of the time?
11
Carbon as the basis of all life Why?
How many bonds can it form with other
molecules? 4 Due to a Carbon having only 4
electrons in its outer shell. What types of
bonds is it going to form most of the
time? Covalent Due to the fact that Carbon is
a non-metal, that will be interacting with other
non-metals
Why is this ideal for biological systems?
12
Carbon as the basis of all life Why?
How many bonds can it form with other
molecules? 4 Due to a Carbon having only 4
electrons in its outer shell. What types of
bonds is it going to form most of the
time? Covalent Due to the fact that Carbon is a
non-metal, that will be interacting with other
non-metals
Why is this ideal for biological systems? -Being
able to make 4 bonds provides the most potential
for diverse molecule structure, and thus, the
highest potential for diverse biological
components. -Covalent bonds are strong when they
need to be long term security of molecule
structure.
13
Organic compounds are constantly being changed
within biological systems! ..You all
probably know this as Metabolism
Metabolism is the constant building and breaking
down of carbon compounds to form the 4 major
types of Organic Compounds
Proteins
Carbohydrates
Lipids Nucleic Acids
14
- Drawing organic compounds for IB
- Easy starting steps
- Draw the carbon atoms in each molecule.
- Add the oxygen (O) or hydroxide (OH).
- Add the nitrogen (N), if it is an amino acid.
- Fill in the bonds in each atom with hydrogen(H)
to make each atom stable
TRY IT WITH THE STRUCTURES BELOW!
Amino Acid
Saturated Fatty Acid
Monomer structures you need to know for IB
15
- Ways to know if a compound is Organic or
Inorganic - Organic compounds contain carbon always.
- There is always at least one C-H bond in an
organic molecule
- Monosaccharide Drawing Practice
- Look up the molecular structures of the three
types of monosaccharides - alpha-glucose
- beta-glucose
- ribose
- And perform the following
- Draw each, using the proper method.
- Look for unique features between each of the
molecules, and circle them on your sheet.
Unique characteristics
Drawing
Title
Set up your sheet to look like this
16
Two major types of metabolic reactions
Anabolism
Catabolism
- the breaking down of complex organic compounds,
resulting in simpler compounds
-the building up of organic compounds from
simpler compounds, resulting in more complex
compounds
AB A B
A B AB
The reaction that performs anabolic action in the
human body is called Condensation
The reaction that performs catabolic action in
the human body is called Hydrolysis.
17
- Condensation reactions make bonds.
- Hydrolysis bonds break these bonds.
- Watch these three animations and make a
- generalization about the processes
- function, reactants, products, role of water
http//is.gd/PeptideBond
http//is.gd/MaltoseGIF
http//is.gd/TriglycerideGIF
18
(No Transcript)
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
(No Transcript)
23
Modelling Exercise Showing Condensation and
Hydrolysis Work with a group to design an
effective model of a monomer, dimer, and polymer.
Using the materials provided, design a mini
sketch, that will display the relationship
between Condensation and Hydrolysis as the
essential reactions for life to occur. Organic
Compounds to choose from Carbohydrates Lipids
Proteins You must have a separate color for
each element, and be able to explain the
following Where water is used Where the
components for water come from Name of
Monomers Name of Dimer
24
(No Transcript)