CHM1C3 - PowerPoint PPT Presentation
1 / 31
Title:
CHM1C3
Description:
... Inductive Effects ... By consideration of inductive effects in structurally related organic acids you should be able to make an ... Nanoscale Chemistry ... – PowerPoint PPT presentation
Number of Views:76
Avg rating:3.0/5.0
Title: CHM1C3
1
Part 2 CHM1C3 Organic Acids and Bases
2
Content of Part 2
Definition of Bronsted acids and
bases Definition of conjugate acids and
bases Ka pKa Typical pKa values Eplaining
differences in acidity Resonance
Effects Eplaining differences in acidity
Inductive Effects
3
CHM1C3 Introduction to Chemical Reactivity of
Organic Compounds
Learning Objectives Part 2 Organic Acids
and Bases
- After completing PART 2 of this course you should
have an understanding of, and be able to
demonstrate, the following terms, ideas and
methods. - (i) You should be able to show the equilibrium
between an organic acid in water with its
conjugate base and the hydroxonium ion. - (ii) You should know what Ka equals with respect
to this equilbrium. - (iii) You should know the relationship between Ka
and pKa. - (v) You should understand that the smaller the
pKa or the more negative the pKa the stronger is
the acid. - By consideration of resonance structures of
structurally related organic acids you should be
able to make an assessment of which structure is
likely to be the most acidic. - (vii) By consideration of inductive effects in
structurally related organic acids you should be
able to make an assessment of which structure is
likely to be the most acidic.
4
(No Transcript)
5
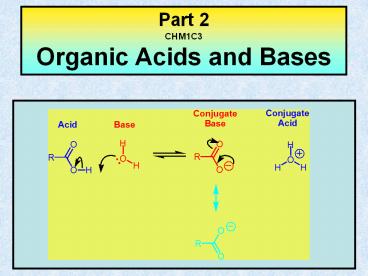
Bronsted Acids and Bronsted Bases
Bronsted Acid A Bronsted acid (HA) is a compound
which acts as a proton donor. Bronted Base A
Bronsted Base (B) is a compound which acts as a
proton acceptor.
Bronsted Acid
Bronsted Base
Conjugate Base
Conjugate Acid
HA B
A BH
6
Examples of Bronsted Acids and Bronsted Bases
Bronsted Acid
Bronsted Base
Conjugate Base
Conjugate Acid
AH B
A BH
CH3CO2H CH3O
CH3CO2 CH3OH
H3O NH3
H2O NH4
H2SO4 H2O
HSO4 H3O
7
Quantifying the Equilibrium Ka
The dissociation of an acid, HA, in water may be
represented as
The water is acting as the base.
Furthermore, the water is acting as the solvent
and is in huge excess.
The degree of ionisation is quantified by the
equilibrium constant
8
Values of Ka
1 Very strong acid Almost complete ionization
large number Approaches infinity
2 Very weak acid No perceptible ionization
small number Approaches zero
9
The pKa
-Log10 Ka
p
Ka pKa Dissociation _at_ 1 mM
1 x 10 3 -3 99.9
1 x 101 -1 92
1 x 100 0 62
1 x 10-1 1 27
1 x 10 -11 11 0.0003
Very strong acid high ionization
Very weak acid low ionization
10
Some Heteroatom pKa Values i.e. atoms attached to
acidic protons other than carbon
STRONG ACID
Acid pKa
HBr -8
HCl -7
H2SO4 -3
HNO3 -1.4
HF 3.18
CF3CO2H 0.23
CCl3CO2H 0.66
NCCH2CO2H 2.47
HCO2H 3.75
Acid pKa
PhCO2H 4.20
CH3CO2H 4.76
(CH3)3CCO2H 5.03
4-nitrophenol 7.15
2-nitrophenol 7.23
3-nitrophenol 8.36
Phenol 10.00
C2H5SH 10.6
CF3CH2OH 12.4
Acid pKa
CH3OH 15.5
C2H5OH 15.9
WEAK ACID
11
Resonance Effects and Acidity
12
Explaining the Differences in Acidity Resonance
Effects
pKa
4.76
Lone pairs of electrons adjacent to double bonds
are able to delocalise through a process referred
to as resonance.
This resonance process imparts stability on the
anionic structure (see Part 1 of the
course) Thus, carboxylate anion is more stable
than the alkoxide anion.
15.5
13
(No Transcript)
14
Explaining the Differences in Acidity Resonance
Effects
pKa 7.23
Stronger Acid
3-Nitrophenol
pKa 8.36
Weaker Acid
15
2-Nitrophenol
Lone pair delocalised into p-system of the
aromatic ring
Lone pair delocalised into p-system of the nitro
group
16
3-Nitrophenol
Lone pair delocalised into p-system of the
aromatic ring
It is not possible for the lone pair to be
positioned on the carbon atom adjacent to the
nitrogen atom.
Therefore, there is one less resonance structure
in this case, and this anion is subsequently less
stable, and more difficult to form from its
protonated form.
17
Explaining the Differences in Acidity Resonance
Effects
pKa 20
Weaker Acid
pKa 9
Stronger Acid
18
An Enolate Dr Coxs Lecture Course
two resonance structures
Less stable anion
three resonance structures
More stable anion
19
Inductive Effects and Acidity
20
Explaining the Differences in Acidity Inductive
Effects
pKa
0.23
3.75
4.20
4.76
5.03
21
This resonance is the same for all the acids
above. Thus, the R groups are influencing the
stability of the carboxylate anion
R affects CO2-
22
CF3 is a strong electron withdrawing group (-I
group) and is pulling electron density away from
the carboxylate, i.e. reducing the charge on the
carboxylate, and thus stabilising it, in a
relative sense.
R CH3 this is a weaker acid. CH3 I
Inductive Group Therefore,
Is a less stable anion.
CH3 is an electron donating group (I group) and
is pushing extra electron density onto the
carboxylate, i.e. increasing the charge on the
carboxylate, and thus destabilising it, in a
relative sense.
23
Some Carbon Atom pKa Values i.e. carbon atoms
attached to acidic protons
VERY WEAK ACID
Acid pKa
(Ph)3CH 31.5
PhCH3 41
Ph-H 43
CH4 48
Cyclohexane 51
Acid pKa
CH3C(O)CH2C(O)CH3 9
CH3NO2 10.2
CH2(CN)2 11.2
Cyclopentadiene 16.0
PhC(O)CH3 19.0
CH3C(O)CH3 20
PhCCH 21
CH3CN 25
HCCH 26
NOT REALLY AN ACID!
24
Summary Sheet Part 2 Organic Acids and Bases
CHM1C3 Introduction to Chemical Reactivity of
Organic Compounds
A Bronsted acid is a compound which can donate a
proton (H). Once the proton has been donated
the resulting structure is referred to as the
conjugate base. A Bronsted base is a compound
which can accept proton. Once the proton has
been accepted the resulting structure is referred
to as the conjugate acid. Any acid/base reaction
is, in principle, an equilibrium process. The
equilibrium can be quantified by considering the
degree of ionisation of an acid dissolved in
water, where the water acts as the Bronsted base.
This quantification is referred to as the pKa
and is equal to the log Ka, where Ka is equal to
the equilibrium concentration of the conjugate
base multiplied by the equilibrium concentration
of the hydroxonium ion divided by the equilibrium
concentration of the Bronsted acid. Consideration
of inductive and resonance effects on the
conjugate base between structurally related
compounds allows a qualitative assessment of the
order of acidity. The more delocalised the lone
pair of electrons (formed from deprotonation of
the acid) the more stable the conjugate base. If
the conjugate base is stabilised, the easier it
will be formed, and thus the stronger the
Bronsted acid will be.
25
www for further pKa information
http//classes.yale.edu/chem220a/studyaids/pKa.htm
l http//www.chromatography.co.uk/TECHNIQS/Other/
buffers.htm http//home.planet.nl/skok/technique
s/laboratory/pka_pkb.html http//www.wiu.edu/user
s/mftkv/Chem331/acidstrength.htm http//www.geoci
ties.com/le_chatelier_uk/pka.html (interesting if
you have audio!) http//www.chem.wisc.edu/areas/r
eich/pkatable/ (pKas in DMSO as
solvent) http//www.agsci.ubc.ca/courses/fnh/410/
protein/1_13.htm (pKas of aminoacids) http//clas
ses.yale.edu/chem220a/studyaids/pKa.html http//w
ww.chem.umd.edu/courses/chem231fribush/3-Chapter2-
3.pdf
26
Question 1 Acids and Bases
Rationalise why acid A is a stronger acid than
acid B.
A, pKa 11.2
B, pKa 25
27
Answer 1 Acids and Bases
Rationalise why acid A is a stronger acid than
acid B.
A, pKa 11.2
B, pKa 25
Most stable anion, as charge more delocalised
over three resonance structures, compared to 2 in
the conjugate base of B. Therefore, A is most
acidic
28
Question 2 Acids and Bases
A and B are two structurally related phenols.
Identify the one which you think will be the most
acidic.
A
B
29
Answer 2 Acids and Bases
A and B are two structurally related benzoic
acids. Identify the one which you think will be
the most acidic.
Two establish which is the strongest acid we need
to consider the conjugate base resonance
structures. We will be able to establish which
has the most resonance structures, and is
therfore the most stable conjugate base and
therefore the most easiest to form.
Most Acidic
A
B
5 Resonance Structures
4 Resonance Structures
30
Question 3 Acids and Bases
A and B are two structurally related phenols.
Identify the one which you think will be the most
acidic.
A
B
31
Answer 3 Acids and Bases
A and B are two structurally related phenols.
Identify the one which you think will be the most
acidic.
Two establish which is the strongest acid we need
to consider the conjugate base resonance
structures. We will be able to establish which
has the most resonance structures, and is
therefore the most stable conjugate base, and
thus the easiest to form.
Most Acidic
A
B
5 Resonance Structures
4 Resonance Structures