Recognition of Molecular Ion - PowerPoint PPT Presentation
Title:
Recognition of Molecular Ion
Description:
... H2O, NH3, C2H4, HCN, CO, H2S,etc often occur Recognition of Molecular Ion Can use CI to verify (usually not convenient) Nitrogen rule: ... – PowerPoint PPT presentation
Number of Views:112
Avg rating:3.0/5.0
Title: Recognition of Molecular Ion
1
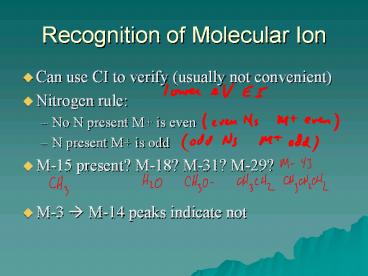
Recognition of Molecular Ion
- Can use CI to verify (usually not convenient)
- Nitrogen rule
- No N present M is even
- N present M is odd
- M-15 present? M-18? M-31? M-29?
- M-3 ? M-14 peaks indicate not
2
Nitrogen Rule
3
Spectrum of an Amine
4
Spectrum of an Alcohol
5
M present?
6
Recognition of Molecular Ion
- Can use CI to verify (usually not convenient)
- Nitrogen rule
- No N present M is even
- N present M is odd
- M-15 present? M-18? M-31? M-29?
- M-3 ? M-14 peaks indicate not
7
Deducing molecular formula
- For small molecules dont need HRMS
- Use m1 to get of Cs
- Use Nitrogen rule
- M-18 peak indicates oxygen
- Doublet M peaks indicate Cl (31) and Br (11)
- Peak at 127 I
- Peak at 77 is phenyl group (C6H5)
- M2 peak 4-5 indicates S
- Deduce molecular formula!
8
Molecular Formula?
9
Molecular Formula?
10
Molecular Formula?
- m/z abund
- 20.2
- 1.2
11
Index of Hydrogen Deficiency
- Units of Unsaturation r db
- CnHmXxNyOz
- r db n m/2 x/2 y/2 1
12
Fragmentation of M
- Draw structure of M if possible and then draw
possible heterolytic cleavages (curved arrows) or
homolytic cleavages (fish hooks) - Alternatively, show homolytic or heterolytic
cleavage cleavage of the molecule and then remove
an electron from one of the fragments. - Fragmentation to give neutral molecule and a
radical cation must involve some kind of
rearrangement.
13
(No Transcript)
14
General Rules
Probablity of bond cleavage depends on stability
of fragments produced. (3º gt 2º gt 1º gt CH3)
(oxonium gt carbocation) (allylic, benzylic gt no
resonance)
- R on cycloalkanes a cleave. Cyclohexenes do retro
Diels-Alder. - R on aromatic rings ß-cleave to benzylic
- Bonds ß to heteroatoms cleave
- Loss of small stable molecules, H2O, NH3, C2H4,
HCN, CO, H2S,etc often occur
- Increase branching ? decrease M
- Add carbons ? decrease M
- Cleave more likely at higher º C
- Unsubst cyclic have strong M (especially
aromatic) - Alkenes cleave to allylic species
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)