Colinearity of Gene and Protein - PowerPoint PPT Presentation
Title:
Colinearity of Gene and Protein
Description:
Colinearity of Gene and Protein DNA genotype DNA sequence transcription RNA translation amino acid sequence protein function organism phenotype – PowerPoint PPT presentation
Number of Views:856
Avg rating:3.0/5.0
Title: Colinearity of Gene and Protein
1
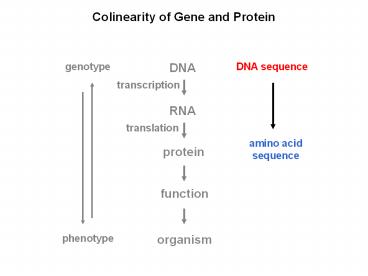
Colinearity of Gene and Protein
DNA
genotype
DNA sequence
transcription
RNA
translation
amino acid sequence
protein
function
organism
phenotype
2
Colinearity of Gene and Protein
The linear sequence of nucleotides in a gene
determines the linear sequence of amino acids in
a protein. Mutant alleles of trpA gene differed
in the position of the mutation at the DNA level,
which corresponded to position of amino acid
substitution in the gene product.
Colinearity of mutations and altered amino acids
in a subunit of tryptophan synthetase from E.
coli C. Yanofsky, 1967. Scientific American
3
Molecular Basis for Relationship between Genotype
and Phenotype
DNA
genotype
DNA sequence
transcription
RNA
translation
amino acid sequence
protein
function
organism
phenotype
4
tRNA
Anticodon of a tRNA molecule recognizes and pairs
with an mRNA codon. tRNA contains modified
bases pseudouridine, methylguanosine,
dimethylguanosine, methylinosine, dihydrouridine.
5
Genetic Code
6
Aminoacyl-tRNA Synthetase Attaches Amino Acid to
tRNA
Aminoacyl-tRNA synthetase catalyzes the formation
of charged tRNA. There is an aminoacyl-tRNA
synthetase for each amino acid. The carboxyl end
of an amino acid is attached to the 3 end of the
tRNA.
7
Wobble Position
Some tRNA molecules can recognize and pair with
more than one specific codon.
Base-pairing between the 3 base of a codon and
5 base of an anticodon is not always exact.
8
Molecular Basis for Relationship between Genotype
and Phenotype
DNA
genotype
DNA sequence
transcription
RNA
translation
amino acid sequence
protein
function
organism
phenotype
9
- Protein Synthesis Brief Summary
- 3 Stages
- Initiation
- Elongation
- Termination
- Catalytic Proteins
- Initiation Factors
- Elongation Factors
- Termination Factors
- Hydrolysis of GTP provides energy to drive some
reactions. - mRNA, rRNA, and tRNA are involved.
10
Protein Synthesis Initiation in Prokaryotes
Shine-Dalgarno sequence pairs with 16S rRNA of
30S subunit. IF3 keeps 30S subunit dissociated
from 50S subunit. Formyl group is added to
methionine when associated with the initiator
tRNA. IF1 and IF2 allows only initiator tRNA to
enter P site. Initiation factors are released
when two ribosomal subunits associate.
11
Protein Synthesis Initiation in
Eukaryotes eIF4A, eIF4B, and eIF4G associates
with 5 end, then with 40S subunit and initiator
tRNA. mRNA is unwound by movement of this
complex in 5 -gt 3 direction. 60S subunit
associates with initiation complex when start
codon is recognized. Initiation factors are
released when the two ribosomal subunits
associate.
12
Important Features of Ribosome
A - aminoacyl site P - peptidyl site E -
exit site
13
Protein Synthesis Elongation
EF-Tu associates with aminoacyl-tRNA to form a
ternary complex.
Correct match of ternary complex with codon in A
site (decoding center) changes conformation of
ribosome. EF-Tu leaves ternary complex, and
peptide bond is formed between amino acids as
amino acids are positioned together in
peptidyltransferase center.
Amino acid in P site is transferred to amino acid
in A site. Translocation requires GTP and EF-G.
EF-G enters A site, shifting tRNAs. When EF-G
leaves, A site is open for a new ternary complex.
A new ternary complex associates with A site,
and deacylated tRNA leaves from E site.
14
Protein Synthesis Termination tRNA molecules do
not recognize stop codons. Termination codons
are recognized by release factors. (RF1, RF2, RF3
in bacteria) UAA and UAG are recognized by
RF1. UAA and UGA are recognized by RF2. RF3
assists in release activity. Release factors
bind to a stop codon in the A site by association
between codon and tripeptide of RF. Polypeptide
is released from P site when RF fits into A
site. Release of polypeptide is followed by
dissociation of ribosomal subunits.
15
Molecular Basis for Relationship between Genotype
and Phenotype
DNA
genotype
DNA sequence
transcription
RNA
translation
amino acid sequence
protein
function
organism
phenotype
16
Molecular Basis for Relationship between Genotype
and Phenotype
DNA
genotype
DNA sequence
transcription
RNA
translation
amino acid sequence
protein
function
organism
phenotype
17
All Protein Interactions in an Organism Compose
the Interactome
Proteome Complete set of proteins produced by
genetic material of an organism. Interactom
e Complete set of protein interactions in an
organism.
18
Alternative Splicing Produces Related but
Distinct Protein Isoforms
19
Posttranslational Events
Protein Folding Translational product
(polypeptide) achieves appropriate folding by aid
of chaperone proteins. Modification of Amino
Acids Phosphorylation/dephosphorylation
Ubiquitination Protein Targeting Directing
proteins to specific locations (for example,
nucleus, mitochondria, or cell membrane) is
accomplished by tagging of proteins (signal
sequence for secreted proteins, nuclear
localization sequences for nuclear proteins).
20
Posttranslational Events
Protein Folding Translational product
(polypeptide) achieves appropriate folding by aid
of chaperone proteins. Modification of Amino
Acids Phosphorylation/dephosphorylation
Ubiquitination Protein Targeting Directing
proteins to specific locations (for example,
nucleus, mitochondria, or cell membrane) is
accomplished by tagging of proteins (signal
sequence for secreted proteins, nuclear
localization sequences for nuclear proteins).
21
Phosphorylation and Dephosphorylation of Proteins
Kinases add phosphate groups to hydroxyl groups
of amino acids such as serine and threonine.
Phosphatases remove phosphate groups.
22
Ubiquitinization Targets a Protein for Degradation
- Short-lived proteins are ubiquitinated
- cell-cycle regulators
- damaged proteins
23
Posttranslational Events
Protein Folding Translational product
(polypeptide) achieves appropriate folding by aid
of chaperone proteins. Modification of Amino
Acids Phosphorylation/dephosphorylation
Ubiquitination Protein Targeting Directing
proteins to specific locations (for example,
nucleus, mitochondria, or cell membrane) is
accomplished by tagging of proteins (signal
sequence for secreted proteins, nuclear
localization sequences for nuclear proteins).
24
Signal Sequences Target Proteins for Secretion
Signal sequence at the amino-terminal end of
membrane proteins or secretory proteins are
recognized by factors and receptors that mediate
transmembrane transport. Signal sequence is
cleaved by signal peptidase.
Nuclear localization sequences (NLSs) are located
in interior of proteins such as DNA and RNA
polymerases. They are recognized by nuclear pore
proteins for transport into nucleus.
25
Universality of Genetic Information
Transfer Genetic code is essentially identical
for all organisms. There are exceptions. System
AUA
UGA universal isoleucine
termination mammalian mitochondria
methionine tryptophan yeast
mitochondria isoleucine
tryptophan
26
Comparison of Gene Expression
Prokaryotes One type of RNA polymerase
synthesizes all RNA molecules. mRNA is
translated during transcription. Genes are not
split. They are continguous segments of
DNA. mRNAs are often polycistronic.
Eukaryotes Three different types of RNA
polymerases synthesize different classes of
RNA. mRNA is processed before translation. Genes
are often split. They are not continguous
segments of coding sequences. mRNAs are mostly
monocistronic.