S298 - PowerPoint PPT Presentation
Title:
S298
Description:
Title: PowerPoint Presentation Author: Chem Last modified by: George Flynn Created Date: 8/11/2003 11:05:33 PM Document presentation format: On-screen Show – PowerPoint PPT presentation
Number of Views:12
Avg rating:3.0/5.0
Title: S298
1
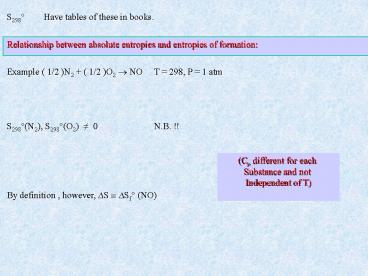
S298 Have tables of these in books.
Relationship between absolute entropies and
entropies of formation
Example ( 1/2 )N2 ( 1/2 )O2 ? NO T 298, P 1
atm
S298(N2), S298(O2) ? 0 N.B. !!
(Cp different for each Substance and not
Independent of T)
By definition , however, ?S ? ?Sf (NO)
2
Also by definition
?Sf (NO) ?Sf(NO) - ( 1/2 ) ?Sf(N2)
?Sf(O2)
?Sf is entropy change in forming a compound from
its elements in their standard state.
S298 is entropy change for any substance going
from T 0 ? T 298
So for a reaction
3
Special case for a substance which has a phase
transition between T of interest and T 0.
e.g. H2O (s, T 0) ? H2O (s, T Tm 273)
?S1
H2O (l, Tm ) ? H2O (l, Tf ) ?S3
4
Cp heat cap H2O (s) ?
Step 1
dqrev Cp dT
Step 3
Cp heat cap H2O (l) ?
Step 2, qrev ?Hfus ( heat of fusion or melting
at constant T Tm)
Solid ? liquid
? ? S3
? ? S1
? ?S2
All liquid
All solid
5
Free Energy
?S 0 for reversible process
But ?S is for system and surroundings (i.e. the
universe)
Would like a function which tells us if a process
is spontaneous or not without referring to
surroundings.
Define new State Function
6
Most common conditions under which chemical
changes occur are P, T consts ?
?G ?H - ?( TS ) ?H - T?S
?G q - T?S
?G q - qrev
7
If a process is reversible q qrev, ?G 0
All at const T, P
Thus, at constant T, P compute ?G for system
alone to determine if a process is
spontaneous/irreversible.
?G gt 0 ? not spontaneous but reverse is!
8
Bonus Bonus Bonus Bonus Bonus Bonus
9
C2H5OH (s) ? C2H5OH (l) P 1 atm
?G gt 0 ? for T (31.5) lt 5012 T lt 159K
?G lt 0 ? for T (31.5) gt 5012 T gt 159K
T 159K is melting pt!
Get irreversible conversion of s ? l above Tm.
Get irreversible conversion to solid
C2H5OH (s) ? C2H5OH (l) P 1 atm
10
Consider freezing of water
H2O (l) ? H2O (s) P 1 atm
?H -6007 joules/mole ?S -21.99
joules/mole-deg
?G ?H - T?S -6007 joules - T (-21.99
joules/deg)
273
?G gt 0 ? for T (21.99) gt 6007 or T gt
273K Water wont freeze Spontaneously!
Water/Ice Equilibrium Point at P1 atm T273
?G lt 0 ? for T (21.99) lt 6007 or T lt
273K Water Freezes Spontaneously
11
Free energy and Equilibrium Constants
Standard free energy of formation, 298K, 1 atm.
( 1/2 )N2 ( 1/2 )O2 NO
?Sf (NO) - ( 1/2 ) ?Sf (N2) - ( 1/2 )?Sf
(O2) ?Sf (NO)
? 0
? 0
?Sf (N2, O2) 0
12
?G, ?H, ?S changes for the reaction
3NO(g)?N2O(g) NO2(g)
However, ?G varies by gt 100 over this range
because the S term is multiplied by T with ?G
?H - T?S
?H varies less than 5 over the range 0-300? C
?S varies only about 5 over the range 0-300? C
13
Competition between ?H and ?S determines sign
of ?G.
?G ?H - T?S
Define T by setting ?G ?H - T?S 0 ? T
?H/ ?S
Both ?H, ?S lt 0, ? ?G lt 0 when Tlt T
Both ?H, ?S gt 0, ? ?G lt 0 when T gt T
14
Standard free energy change for a reaction
aA bB ? cC dD
?G c?Gf(C) d?Gf(D) - a?Gf(A) - b?Gf(B)
i.e. mixture of a of A, b of B, etc, goes
spontaneously to right
Must be careful. Does not imply complete
conversion from reactants to products.
15
Need to know how ?G depends on concentration.
Concentration Dependence for Simplest Case
1 mole , ideal gas, constant T, but not const P.
(Note P defines concentration)
but ?E 0 since T constant and ?(PV) ?(RT)
0
16
?G - qrev
However since ?E q w 0, q -w
?G wrev (Free energy related to reversible
work)
Because work is reversible
17
If we define Gi G ( Pi 1 atm ) then
where Pf must be expressed in atmospheres.
In general for 1 mole ideal gas
Shows how G depends on P at fixed T if know G(T).
To distinguish G for 1 mole, call it ? (T, P)
? (T) RT ln P