Propylene Sulfide - C2H4S - PowerPoint PPT Presentation
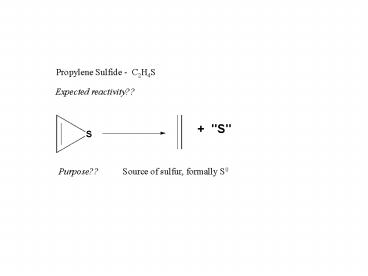
Propylene Sulfide - C2H4S
Title: PowerPoint Presentation Author: Sharon Burgmayer Last modified by: Bryn Mawr College Created Date: 11/6/2006 11:19:37 PM Document presentation format – PowerPoint PPT presentation
Title: Propylene Sulfide - C2H4S
1
Propylene Sulfide - C2H4S
Expected reactivity??
Purpose??
Source of sulfur, formally S0
2
What happens in reactions with Mo complexes?
?
S
- characteristics
- Mo(4) could be oxidized or reduced
- open (vacant) coordination site
- Mo loves S
- characteristics
- Mo(6)
- filled coordination sphere
- Mo loves S
- Seems OK but is it right?
How could we know for sure?????
3
Before deciding, you need to know about sulfur
Sulfur is strange!!!! Thats why the alchemists
loved it. They thought that everything, every
substance could be made from the proper mixture
of sulfur, mercury and salt.
Sulfur does not behave like its smaller cousin, O.
As ions S2- S22- O2- O22-
O2 - polysulfides S32- S52-
4
Infrared Spectroscopy helps assign sulfur ligand
type MS has nMS 450-500 cm-1 M(S2) has
nM-S 500-550 cm-1 M(S3), M(S4) has nM-S lt
480 cm-1
Lets try it
5
TpMo(X)(S4)
What is X?
6
compound was determined to be TpMo(S)(S4)
7
Product was determined to be a mixture of
both TpMo(S)(S4) and TpMo(O)(S4)
MoS
MoO
8
Whats the difference between pictures A and B?
A
B
9
UV/vis Spectroscopy Electronic
Spectroscopy What does it monitor / probe? What
information about molecule can one obtain?
10
1H NMR what do you expect to see?
11
(No Transcript)
12
(No Transcript)
13
a
a
b
b
c
c
Expected 3 resonances, 2 -CH3 (a c with 6H)
and 1 H (b) ( B-H not visible due
to broadening) plus Hs on cation TEA
N(CH2CH3)4
14
1H NMR what do you expect to see?
TpMo(S)(S4)
15
(No Transcript)
16
MoS
MoO
TEA -CH3
TEA -CH2-
17
d
e
MoS
MoO
f
a
b
c
a
TEA -CH3
b
c
TEA -CH2-
a
c
b
d
e
f
18
R1 ? R2 How many Hs expected in NMR from
pyrazole rings?
All -methyl and ring Hs will be unique see 6
-Me and 3 ring H
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.