1.Diffraction of light - PowerPoint PPT Presentation
1 / 9
Title:
1.Diffraction of light
Description:
Wave-particle duality of light Evidence of the wave nature of light: 1. Diffraction of light Light diffracts when it passes the edge of a barrier or passes through a ... – PowerPoint PPT presentation
Number of Views:115
Avg rating:3.0/5.0
Title: 1.Diffraction of light
1
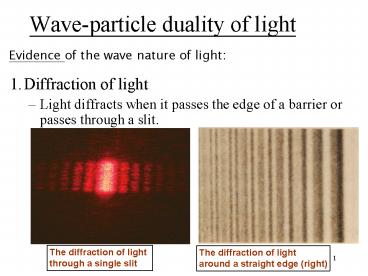
Wave-particle duality of light
Evidence of the wave nature of light
- 1. Diffraction of light
- Light diffracts when it passes the edge of a
barrier or passes through a slit.
The diffraction of light through a single slit
The diffraction of light around a straight edge
(right)
2
- 2. Interference of light
- Light produces an interference pattern when
passed through a double slit.
Youngs double slit experiment
3
Evidence of the particle nature of light
- 1. The photoelectric effect
- The phenomenon could be readily explained using
the concept of the photon but not the wave theory
of light.
The photoelectric effect
4
- 2. Compton scattering
- When a high-energy EM wave is directed to a free
electron, the scattered wave has a lower
frequency. - Hence a photon carries not only energy but also
momentum, just as particles do.
Collision between two billiard balls
Compton scattering
5
Matter waves
The de Broglie theory
- De Broglie proposed that a matter particle or
object had an associated matter wave.
- The de Broglie wavelength of a matter particle
(wave) is - h the Planck constant
- p momentum of the particle.
Like light, electrons also demonstrate
wave-particle duality.
6
The de Broglie wavelength and the wave nature of
matter
- The wave nature of macroscopic objects is
unobservable because their de Broglie wavelengths
are too short.
The wavelength of a moving volleyball is much
shorter than any known dimension in daily life.
The wavelength of electrons is comparable to the
interatomic spacing in crystals.
7
Electron diffraction the evidence of matter waves
The electron diffraction experiment by George
Thomson
Diffraction rings formed by a beam of electrons
(left) and a beam of X-rays (right) through the
same metal foil
8
Electron interference
- The most important evidence of the wave nature of
light is obtained from Youngs double slit
experiment.
Interference fringes produced by light
- Similar interference experiments have been
carried out with electrons as well.
Interference fringes produced by electron
9
Implications of the wave-particle duality
- Electrons behave like waves when they pass
through gaps of small enough sizes, but behave
like particles when they interact with matter. - Hence electron exhibits wave-particle duality
just as light does. - This also tells us that the microscopic world is
very different from the macroscopic world we see
in daily life.
Electrons pass through small gaps in a wave-like
manner but hit the screen in a particle-like
manner.