Double Displacement (or Replacement) Reactions - PowerPoint PPT Presentation
Title:
Double Displacement (or Replacement) Reactions
Description:
Double Displacement (or Replacement) Reactions Also referred to as metathesis reaction The two compounds exchange ions to produce two new compounds. – PowerPoint PPT presentation
Number of Views:122
Avg rating:3.0/5.0
Title: Double Displacement (or Replacement) Reactions
1
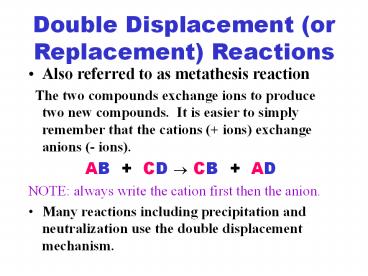
Double Displacement (or Replacement) Reactions
- Also referred to as metathesis reaction
- The two compounds exchange ions to produce two
new compounds. It is easier to simply remember
that the cations ( ions) exchange anions (-
ions). - AB CD ? CB AD
- NOTE always write the cation first then the
anion. - Many reactions including precipitation and
neutralization use the double displacement
mechanism.
2
Double Displacement (or Replacement) Reactions
- PREDICT THE PRODUCT BALANCE
- 1. MgSO4 LiOH ? ___________
- 2. Pb(NO3)2 Na2CO3 ? ____________
- 3. HNO3 Ba(OH)2 ? ___________
Answers are on the next slide.
3
Double Displacement (or Replacement) Reactions
- ANSWERS
- 1. MgSO4 2 LiOH ? Mg(OH)2 Li2SO4
- 2. Pb(NO3)2 Na2CO3 ? PbCO3 2 NaNO3
- 3. 2 HNO3 Ba(OH)2 ? Ba(NO3)2 2 H2O
Exchange cations
4
PRECIPITATION REACTION
- A reaction where an insoluble solid is formed
during a reaction between two aqueous solutions. - (aq) (aq) ? (aq) (s)
- 2KI(aq) Pb(NO3)2(aq) ? 2KNO3(aq) PbI2(s)
- NEUTRALIZATION REACTION
- A reaction between an acid and a base which
results in the production of a salt and water. - HA BOH ? (metal/nonmetal) H2O
- HNO3(aq) KOH(aq) ? KNO3(aq) H2O(l)
Exchange cations
5
Single Displacement (or Replacement) Reactions
- One element reacts with a compound to produce a
different element and a new compound. - A BC ? AC B
- NOTE if the element is a metal, it will replace
the cation. - A BC ? C BA
- NOTE if the element is a nonmetal, it will
replace the anion. - Many reduction-oxidation reactions use the
single displacement mechanism.
6
Single Displacement (or Replacement) Reactions
- PREDICT THE PRODUCT
- 1. Ca HCl ?
- 2. ZnBr2 I2 ?
- 3. Cu AgNO3 ?
Answers are on the next slide.
7
Single Displacement (or Replacement) Reactions
- ANSWERS
- 1. Ca 2 HCl ? CaCl2 H2
- 2. ZnBr2 I2 ? ZnI2 Br2
- 3. Cu 2AgNO3 ? 2Ag Cu(NO3)2
8
Reduction-Oxidation Reactions
- A reaction in which electrons are transferred
from one species to another. - Oxidation means the loss of electrons
- Reduction means the gain of electrons
- Rusting is a redox reaction
- 4Fe (s) 3O2 (g) ? 2Fe2O3 (s)
- As a reactant Fe has a zero oxidation state but
as a product (in Fe2O3) iron has a 3 oxidation
state. Three electrons per atom had to be
transferred (lost) in order for this to happen.
Note that oxygen also changed from a zero
oxidation state to a 2- oxidation state. Oxygen
needed to gain 2 electrons per atom.
9
Reduction-Oxidation Reactions
- Oxidation means the loss of electrons
- Reduction means the gain of electrons
- Electrochemistry involves redox Rx.
- Cu(s) 2AgNO3(aq) ? 2Ag(s) Cu(NO3)2(aq)
- The reactant Cu has a zero oxidation state (all
elements have a zero oxidation state) but as a
product, in Cu(NO3)2,the copper atom loses two
electrons and has a 2 oxidation state. The
other atom which acquired the electrons donated
by copper is silver. As a reactant silver has a
1 oxidation state then by gaining electrons from
copper, the ions are turned into elemental silver
with a zero oxidation state. - The net effect of this reaction has metallic
copper being oxidized to copper ions and silver
ions being reduced to silver metal.
10
COMBINATION REACTION
- A reaction in which two or more substances
combine to form a single product. - A B C ? ABC
- CaO(s) SO2(g) ? CaSO3(s)
- DECOMPOSITION REACTION
- A reaction in which a single compound reacts to
give two or more substances, usually requiring a
raise in temperature. - ABC ? A B C
- 2KClO3(s) ? 2KCl(s) 3O2(g)
11
COMBUSTION REACTION
- A reaction of a substance with oxygen, usually
the rapid release of heat produces a flame. - CH O2 ? CO2 H2O
- 2C4H10(g) 13O2(g) ? 8CO2(g) 10H2O(g)
- Many times in a combustion reaction, heat energy
is given off. In chemical terms this is called
an exothermic reaction. Thermochemistry is field
of chemistry which studies the transfer of heat
in a reaction. - The thermodynamic equation representing this
exothermic reaction is - 2C4H10(g) 13O2(g) ? 8CO2(g) 10H2O(g)
heat (in Joules)
12
GAS FORMATION REACTIONS
- A reaction that produces a gas from reactants not
in the gaseous state. - 2 HCl (aq) ZnS (s) ? ZnCl2 (aq) H2S (g)
- Zn (s) 2 HCl (aq) ? ZnCl2 (aq) H2 (g)
- Many gas formation reactions involve two steps,
first the double displacement reaction then the
decomposition reaction of an unstable substance.
- Na2CO3 2HCl ? 2 NaCl H2CO3
- H2CO3 ? CO2 H2O
- Besides carbonic acid (H2CO3), sulfurous acid
(H2SO3) also decomposes into SO2 and water.
13
PRACTICE PROBLEMS Write the following as
balanced chemical equations then classify each
reaction.
- 1. Magnesium metal is combined with nitrogen at
elevated temperatures to form magnesium nitride
powder. - 2. An aqueous solution of soluble aluminum
nitrate is mixed with aqueous sodium hydroxide to
produce insoluble aluminum hydroxide and a sodium
nitrate solution. - 3. Solid potassium sulfite is added to
hydrochloric acid to produce sulfur dioxide,
water, and potassium chloride. - 4. Acetic acid reacts with calcium hydroxide to
produce calcium acetate and water. - 5. Lithium metal is dropped in water to produce
lithium hydroxide and hydrogen gas.
3 Mg (s) N2 (g) ? Mg3N2 (s) combination, redox
Al(NO3)3 (aq) 3NaOH (aq) ? Al(OH)3(s)
3NaNO3(aq) ppt, DD
K2SO3 (s) 2HCl (aq) ? H2SO3 (aq) 2KCl (aq)
DD then H2SO3 (aq) ? H2O (l) SO2 (g)
decomposition overall Rx K2SO3 (s) 2HCl (aq)
? H2O (l) SO2 (g) 2KCl (aq)
2 HC2H3O2 (aq) Ca(OH)2 (aq) ? Ca(C2H3O2)2 (aq)
2H2O (l) DD, neutralization
2Li (s) 2H2O(l) ? 2LiOH (aq) H2 (g) SD, redox
14
GROUP STUDY PROBLEMSWrite the following as
balanced chemical equations then classify each
reaction.
- 1. Solid mercuric oxide decomposes at high
temperatures to form metallic mercury and oxygen. - 2. Aqueous lead(II) nitrate reacts with aqueous
magnesium bromide to produce the insoluble salt
lead(II)bromide and soluble magnesium nitrate. - 3. At room temperature, aqueous ammonium
carbonate is added to hydrochloric acid to
produce aqueous ammonium chloride, water and
carbon dioxide. - 4. Hydrochloric acid is poured over tin metal
producing hydrogen gas and tin (IV) chloride. - 5. The combustion of ethanol, C2H5OH, results in
the production of carbon dioxide and water.