Projected Trends in U.S. Liquid Fuels Demand - PowerPoint PPT Presentation
1 / 12
Title:
Projected Trends in U.S. Liquid Fuels Demand
Description:
Projected Trends in U.S. Liquid Fuels Demand With world oil prices in the range of 15-25 USD/bbl, liquid fuel chemistry is the single most valuable catalytic ... – PowerPoint PPT presentation
Number of Views:78
Avg rating:3.0/5.0
Title: Projected Trends in U.S. Liquid Fuels Demand
1
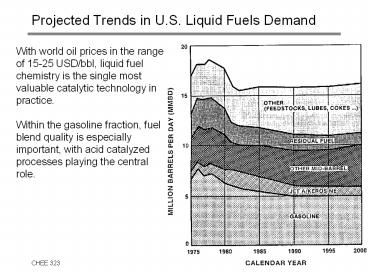
Projected Trends in U.S. Liquid Fuels Demand
- With world oil prices in the range of 15-25
USD/bbl, liquid fuel chemistry is the single most
valuable catalytic technology in practice. - Within the gasoline fraction, fuel blend quality
is especially - important, with acid catalyzed processes playing
the central - role.
2
Gasoline Blend Formulations
3
Performance Value of Gasoline Components
4
Refinery Processes - Applications of Acid
Catalysis
5
Acid Catalysis vs. Metal/Organometallic Catalysis
- Where selectivity is concerned, using acid
catalysis is akin to performing surgery with farm
implements. - wide range of products accessible from carbenium
ion intermediates - selectivity for given compounds is very often
quite poor - Why acid catalysis for fuel production?
- Relatively cheap initiators compared to
organometallic compounds - Solid superacids allow for heterogeneous,
gas-phase chemistry - Fuel performance is insensitive to structure in
comparison with drug action, polymer performance.
6
Estimated Refining Catalysts/Usage in 1987
7
General Kinetics of Acid Catalyzed Processes
- The acid catalyzed processes utilized for fuel
production can be classified as truly catalytic
(closed sequences without loss of active species)
or as chain reactions (closed sequences requiring
initiation and experiencing large-scale
deactivation). - Processes generally involve
- 1. Carbenium ion generation
- 2. Transformation
- olefin isomerization
- rearrangement
- alkylation
- fragmentation (cracking)
- hydride abstraction
- 3. Chain processes terminate by
- hydride abstraction
- collapse of ion pair
8
Carbenium Ion Generation
- 1. Protonation of an unsaturated hydrocarbon
- If the olefin is not strongly basic, a strong
acid is required to generate the carbocation - 2. Protonation of a saturated hydrocarbon
- predominant mechanism in the cracking of alkanes
- carbonium ion
- 3. Electron removal from a neutral molecule
9
Basicity of Unsaturated Hydrocarbons
- The ease of substrate protonation, while easily
measured for relatively good bases (ketones,
amines, etc), is difficult to estimate for
hydrocarbons. - Theoretical estimates of proton affinity for
various olefins point to increasing basicity with
increased carbenium ion substitution. - Direct measurements of these equilibria are not
possible, because several catalytic reactions
(polymerization, isomerization, hydride transfer)
take place as soon as the carbenium ion is formed
in strongly acidic media.
10
Superacids
- Combinations of Lewis and Bronsted acids can
create extremely acidic conditions that are
needed for hydrocarbon conversions. - Acid Ho
- HSO3F -12.8
- BF3 / H2O -11.4
- H2SO4 (98) -9.36
- HF -10.2
- BF3 / HF -16.8
- TaF5 (10mol) / HF -18.9
- SbF5 (10mol) / HF -18.9
- SbF5 / HSO3F -18.9
- The term Friedel-Crafts Acids is often used.
These are metal halides, organometal halides or
organometals that coordinatively stabilize
conjugate bases (anions, conjugate bases of
Bronsted acids, halogens) by complexation. AlCl3
activated by HCl is the best known example.
11
Relative Stability of Alkyl Cations
- The stability of alkyl cations is influenced by
- inductive effects (substituents)
- hyperconjugation
12
Hammonds Postulate
- The transition state for alkene protonation
closely resembles the carbocation. Therefore,
the factors that contribute to the relatively
high energy of the carbocation contribute to the
instability of the transition state. - Hammonds Postulate is the assumption that the
transition state for the formation of reactive
intermediates resembles the intermediates
themselves. - Protonation of isobutylene to
- give the tertiary carbocation
- has the more stable transition
- state, and is therefore the
- kinetically preferred reaction
- pathway.