Harmonic Oscillator - PowerPoint PPT Presentation
Title:
Harmonic Oscillator
Description:
Harmonic Oscillator Harmonic Oscillator IR spectroscopy is an important tool in structural determination of unknown compound CO2, A greenhouse gas ? – PowerPoint PPT presentation
Number of Views:238
Avg rating:3.0/5.0
Title: Harmonic Oscillator
1
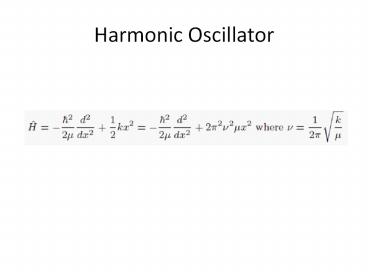
Harmonic Oscillator
2
Harmonic Oscillator
3
Selections rules
Permanent Dipole moment
An electric dipole consists of two electric
charges ?q and -?q separated by a distance R.
This arrangement of charges is represented by a
vector, the electric dipole moment ? with a
magnitude
? Re ?q Unit Debye, 1D
3.3310-30Cm When the molecule is at its
equilibrium position, the dipole moment is called
permanent dipole moment ?0.
4
Selections rules Electric dipole moment operator
? The probability for a vibrational transition to
occur, i.e. the intensity of the different lines
in the IR spectrum, is given by the transition
dipole moment ?fi between an initial vibrational
state ?i and a vibrational final state ?f
The electric dipole moment operator depends on
the location of all electrons and nuclei, so its
varies with the modification in the
intermolecular distance x. ?0 is the permanent
dipole moment for the molecule in the equilibrium
position Re
5
0
The higher terms can be neglected for small
displacements of the nuclei
The two states ?i and ?f are orthogonal. Because
they are solutions of the operator H which is
Hermitian
6
Second condition
First condition ?fi 0, if ??/ ?x 0
In order to have a vibrational transition visible
in IR spectroscopy the electric dipole moment of
the molecule must change when the atoms are
displaced relative to one another. Such
vibrations are infrared active. It is valid
for polyatomic molecules.
By introducing the wavefunctions of the initial
state ?i and final state ?f , which are the
solutions of the SE for an harmonic oscillator,
the following selection rules is obtained ?? 1
7
Note 1 Vibrations in homonuclear diatomic
molecules do not create a variation of ? ? not
possible to study them with IR spectroscopy. Note
2 A molecule without a permanent dipole moment
can be studied, because what is required is a
variation of ? with the displacement. This
variation can start from 0.
8
IR Stretching Frequencies of two bonded atoms
What Does the Frequency, ?, Depend On?
- frequency
- k spring strength (bond stiffness)
- ? reduced mass ( mass of largest atom)
- is directly proportional to the strength of the
bonding between the two atoms (? ? k) - is inversely proportional to the reduced mass
of the two atoms (v ? 1/?)
51
9
Stretching Frequencies
- Frequency decreases with increasing atomic
weight. - Frequency increases with increasing bond energy.
52
10
IR spectroscopy is an important tool in
structural determination of unknown compound
11
IR Spectra Functional Grps
Alkane
C-C
-C-H
Alkene
Alkyne
11
12
IR Aromatic Compounds
(Subsituted benzene teeth)
CC
12
13
IR Alcohols and Amines
O-H broadens with Hydrogen bonding
CH3CH2OH
C-O
N-H broadens with Hydrogen bonding
Amines similar to OH
13
14
CO2, A greenhouse gas ?
15
Electromagnetic Spectrum
- Over 99 of solar radiation is in the UV,
visible, and near infrared bands - Over 99 of radiation emitted by Earth and the
atmosphere is in the thermal IR band (4 -50 µm)
Near Infrared
Thermal Infrared
16
What are the Major Greenhouse Gases?
- N2 78.1
- O2 20.9
- H20 0-2
- Ar other inert gases 0.936
- CO2 370ppm
- CH4 1.7 ppm
- N20 0.35 ppm
- O3 10-8
- other trace gases
17
Molecular vibrations
- The lowest vibrational transitions of diatomic
molecules approximate the quantum harmonic
oscillator and can be used to imply the bond
force constants for small oscillations. - Transition occur for ?v 1
- This potential does not apply to energies close
to dissociation energy. - In fact, parabolic potential does not allow
molecular dissociation. - Therefore more consider anharmonic oscillator.
18
Vibrational modes of CO2
19
Anharmonic oscillator
- A molecular potential energy curve can be
approximated by a parabola near the bottom of the
well. The parabolic potential leads to harmonic
oscillations. - At high excitation energies the parabolic
approximation is poor (the true potential is less
confining), and does not apply near the
dissociation limit. - Must therefore use a asymmetric potential. E.g.,
The Morse potential - where De is the depth of the potential minimum
and
20
Anharmonic oscillator
- The Schrödinger equation can be solved for the
Morse potential, giving permitted energy levels - where xe is the anharmonicity constant
- The second term in the expression for E increases
- with v gt levels converge at high quantum
numbers. - The number of vibrational levels for a Morse
- oscillator is finite
- v 0, 1, 2, , vmax
21
Energy Levels Basic Ideas
Basic Global Warming The C02 dance
About 15 micron radiation