Chapter%2023%20 - PowerPoint PPT Presentation
Title:
Chapter%2023%20
Description:
Chapter 23 Functional Groups Pre-AP Chemistry Charles Page High School Stephen L. Cotton – PowerPoint PPT presentation
Number of Views:413
Avg rating:3.0/5.0
Title: Chapter%2023%20
1
Chapter 23Functional Groups
- Pre-AP Chemistry
- Charles Page High School
- Stephen L. Cotton
2
Section 23.1 - Introduction to Functional Groups
- OBJECTIVES
- Explain how organic compounds are classified.
3
Section 23.1 - Introduction to Functional Groups
- OBJECTIVES
- Identify halocarbons and the IUPAC rules for
naming halocarbons.
4
Section 23.1 - Introduction to Functional Groups
- OBJECTIVES
- Describe how halocarbons can be prepared.
5
Functional Groups
- Most organic chemistry involves substituents
- often contain O, N, S, or P
- also called functional groups- they are the
chemically functional part of the molecule, and
are the non-hydrocarbon part
6
Functional Groups
- Functional group - a specific arrangement of
atoms in an organic compound, that is capable of
characteristic chemical reactions. - What is the best way to classify organic
compounds? By their functional groups.
7
Functional Groups
- The symbol R is used to represent any carbon
chains or rings - Important Table 23.1, page 726 -- shows some of
the major categories, and their functional groups
- KNOW THESE. - Table 23.2, p. 727 - alkyl groups
8
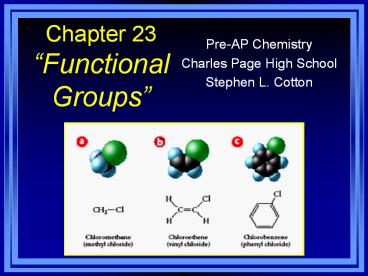
Halogen Substituents
- Halocarbons - class of organic compounds
containing covalently bonded fluorine, chlorine,
bromine, or iodine - General formula R-X (X halogen)
- Naming? Name parent as normal, add the halogen
as a substituent (or prefix) - Examples on page
726
9
Halogen Substituents
- Common namesp.726
- The more highly halogenated the compound is, the
higher the b.p. (see Table 23.3, page 728) - Few halocarbons found in nature
- but, readily prepared and used
- halothane (Fig. 23.3, p.727) and also the
hydrofluorocarbons
10
Substitution Reactions
- Organic reactions often much slower than
inorganic reactions - must break strong covalent bond
- trying to find new catalysts to use
- Substitution - an atom (or group of atoms)
replaces another atom or group of atoms
11
Substitution Reactions
- A halogen (shown as X) can replace a hydrogen
to make a halocarbon - R-H X2 ? R-X HX
- Sunlight is often a sufficient catalyst
- CH4 Cl2 ? CH3Cl HCl
UV light
12
Substitution Reactions
- Treating benzene with a halogen? Examples on Page
729 - Halogens on carbon chains are readily displaced
by hydroxide ions (OH1-) to make an alcohol a
salt - R-X OH1- ? R-OH X1-
- CH3-Cl NaOH ? CH3-OH NaCl
Methanol sodium chloride
13
Substitution Reactions
- CH3-I KOH ? CH3-OH KI
- CH3CH2Br NaOH ? CH3CH2OH NaBr
Iodomethane
Methanol
Bromoethane
Ethanol
14
Section 23.2Alcohols and Ethers
- OBJECTIVES
- Identify how alcohols are classified and named.
15
Section 23.2Alcohols and Ethers
- OBJECTIVES
- Predict how the solubility of an alcohol varies
with the length of its carbon chain.
16
Section 23.2Alcohols and Ethers
- OBJECTIVES
- Name the reactions of alkenes that may be used to
introduce functional groups.
17
Section 23.2Alcohols and Ethers
- OBJECTIVES
- Construct the general structure of an ether and
describe how ethers are named.
18
Alcohols
- Alcohols - a class of organic compounds with an
-OH group - The -OH functional group in alcohols is called a
hydroxyl group thus R-OH is the formula - How is this different from the hydroxide ion?
(covalent bonding with the carbon- not ionic with
a metal like bases)
19
Alcohols
- Aliphatic alcohols classified into categories
according to the number of R groups attached to
the carbon with the hydroxyl - 1 R group primary alcohol
- 2 R groups secondary alcohol
- 3 R groups tertiary alcohol
- Note drawings on page 730
20
Alcohols
- Both IUPAC and common names
- For IUPAC
- drop the -e ending of the parent alkane name add
ending of -ol, number the position of -OH - parent is the longest chain that contains the
carbon with the hydroxyl attached.
21
Alcohols
- The hydroxyl is given the lowest position number
- Alcohols containing 2, 3, and 4 of the -OH
substituents are named diols, triols, and tetrols
respectively - Examples on page 731
22
Alcohols
- Common names
- similar to halocarbons, meaning name the alkyl
group, then followed by the word alcohol - One carbon alcohol methyl alcohol
23
Alcohols
- More than one -OH substituents are called glycols
(ethylene glycol?) - Examples on page 731
- Phenols - compounds in which a hydroxyl group is
attached directly to an aromatic ring. Cresol is
the common name of o, m, and p isomers of
methylphenol
24
Properties of Alcohols
- Much like water, alcohols are capable of hydrogen
bonding between molecules - this means they will boil at a higher temp. than
alkanes and halocarbons with a comparable number
of atoms
25
Properties of Alcohols
- Alcohols are derivates of water the -OH comes
from water, and thus are somewhat soluble - Alcohols of up to 4 carbons are soluble in water
in all proportions more than 4 carbons are
usually less soluble, because the longer carbon
chain is more nonpolar
26
Properties of Alcohols
- Many aliphatic alcohols used in laboratories,
clinics, and industry - Isopropyl alcohol (2-propanol) is rubbing
alcohol used as antiseptic, and a base for
perfume, creams, lotions, and other cosmetics - Ethylene glycol (1,2-ethanediol) - commonly sold
as antifreeze
27
Properties of Alcohols
- Glycerol (1,2,3-propanetriol) - used as a
moistening agent in cosmetics, foods, and drugs
also a component of fats and oils - Ethyl alcohol (ethanol) used in the intoxicating
beverages also an important industrial solvent
28
Properties of Alcohols
- Denatured alcohol- means it has been made
poisonous by the addition of other chemicals,
often methyl alcohol (methanol, or wood alcohol). - As little as 10 mL of methanol has been known to
cause permanent blindness, and 30 ml has resulted
in death!
29
Addition Reactions
- The carbon-carbon single bond is not easy to
break - In double bonded alkenes, it is easier to break a
bond - Addition reaction- substance is added at the
double or triple bond location, after it is broken
30
Addition Reactions
- Addition of water to an alkene is a hydration
reaction - usually occurs with heat and an acid
(such as HCl or H2SO4 acting as a catalyst) - Note sample at top of page 734 for the formation
of ethanol from ethene water
31
Addition Reactions
- If a halogen is added in an addition reaction,
the result is a halocarbon that is disubstituted
- middle p. 734 - The addition of bromine is often used as a test
for saturation - p.734 - Addition of a hydrogen halide? -called
monosubstituted halocarbon
32
Addition Reactions
- Addition of hydrogen to produce an alkane is a
hydrogenation reaction, which usually involves a
catalyst such as Pt or Pd - common application is the manufacture of
margarine from unsaturated vegetable oils (making
them solid from a liquid)
33
Addition Reactions
- The hydrogenation of a double bond is a reduction
reaction, which in one sense is defined as the
gain of H - Top- page 735, ethene is reduced to ethane
cyclohexene is reduced to cyclohexane
34
Ethers
- A class of organic compounds in which oxygen is
bonded to 2 carbon groups R-O-R is formula - Naming? The two R groups are alphabetized, and
followed by ether - Two R groups the same? Use the prefix di-
Examples on page 735
35
Ethers
- Diethyl ether is the one commonly called just
ether - was the first reliable general anesthetic
- dangerous- highly flammable, also causes nausea
- ethers are fairly soluble in water
- Alcohol used for fuel in the future?
36
Section 23.3Carbonyl Compounds
- OBJECTIVES
- Identify the structure of a carbonyl group as
found in aldehydes and ketones.
37
Section 23.3Carbonyl Compounds
- OBJECTIVES
- Construct the general formula for carboxylic
acids and explain how they are named.
38
Section 23.3Carbonyl Compounds
- OBJECTIVES
- Describe an ester.
39
Section 23.3Carbonyl Compounds
- OBJECTIVES
- Explain how dehydrogenation is an oxidation
reaction.
40
Aldehydes and Ketones
- Review
- alcohol has an oxygen bonded to a carbon group
and a hydrogen - ether has an oxygen bonded to two carbon groups
- An oxygen can also be bonded to a single carbon
by a double bond
41
Aldehydes and Ketones
- The CO group is called the carbonyl group
- it is the functional group in both aldehydes and
ketones - Aldehydes - carbonyl group always joined to at
least one hydrogen (meaning it is always on the
end!)
42
Aldehydes and Ketones
- Ketones - the carbon of the carbonyl group is
joined to two other carbons (meaning it is never
on the end) - Structures - bottom of page 737
43
Aldehydes and Ketones
- Naming?
- Aldehydes identify longest chain containing the
carbonyl group, then the -e ending replaced by
-al, such as methanal, ethanal, etc. - Ketones longest chain w/carbonyl, then new
ending of -one number it? - propanone, 2-pentanone, 3-pentanone
44
Aldehydes and Ketones
- Table 23.4, page 738 examples
- Neither can form intermolecular hydrogen bonds,
thus a much lower b.p. than corresponding
alcohols - wide variety have been isolated from plants and
animals possible fragrant odor or taste many
common names
45
Aldehydes and Ketones
- Benzaldehyde
- Cinnamaldehyde
- Vanillin
- Methanal (the common name is formaldehyde)
- 40 in water is formalin, a preservative
46
Aldehydes and Ketones
- Propanone (common acetone) is a good solvent
miscible with water in all proportions - why is it a good substance used in nail-polish
removers? (a powerful solvent-able to dissolve
both polar nonpolar)
47
The Carboxylic Acids
- Also have a carbonyl group (CO), but is also
attached to a hydroxyl group (-OH) carboxyl
group - general formula R-COOH
- weak acids (ionize slightly)
- Named by replacing -e with -oic and followed by
the word acid - methanoic acid ethanoic acid
48
Carboxylic Acids
- Abundant and widely distributed in nature, many
having a Greek or Latin word describing their
origin - acetic acid (ethanoic acid) from acetum, meaning
vinegar - many that were isolated from fats are called
fatty acids - Table 23.6 page 741
49
The Esters
- General formula RCOOR
- Derivatives of the carboxylic acids, in which the
-OH from the carboxyl group is replaced by an -OR
from an alcohol - carboxylic acid alcohol ? ester water
- many esters have pleasant, fruity odors- banana,
pineapple, perfumes
50
Esters
- Although polar, they do not form hydrogen bonds
(reason there is no hydrogen bonded to a highly
electronegative atom!) - thus, much lower b.p. than the hydrogen-bonded
carboxylic acids they came from
51
Esters
- Can be prepared from a carboxylic acid and an
alcohol usually a trace of mineral acid added as
catalyst (because acids are dehydrating agents) - Note equation on bottom p. 742
52
Esters
- Naming? It has 2 words
- 1st alkyl attached to single bonded oxygen from
alcohol - 2nd take the acid name, remove the -ic acid, add
-ate - example on top of page 743
53
Oxidation- Reduction Reactions
- All of the previous classes of organic compounds
are related by oxidation and reduction reactions - What is oxidation-reduction?
- Oxidation the gain of oxygen, loss of hydrogen,
or loss of e-1 - Reduction the loss of oxygen, gain of hydrogen,
or gain of e-1
54
Oxidation- Reduction Reactions
- Oxidation and reduction reactions (sometimes
called redox) are coupled- one does not occur
without the other - The number of Oxygen and Hydrogen attached to
Carbon indicates the degree of oxidation
55
Oxidation- Reduction Reactions
- The fewer the of H on a C-C bond, the more
oxidized the bond - Thus, a triple bond is more oxidized than a
double bond and a single bond - An alkane is oxidized (loss of H) to an alkene,
and then to an alkyne
56
Oxidation- Reduction Reactions
- Loss of hydrogen is called a dehydrogenation
reaction - may require strong heating and a catalyst
- Note equations at the top on page 744
57
Oxidation- Reduction Reactions
- Methane can be oxidized in steps to carbon
dioxide (middle p. 744) - methane ? methanol ? methanal ? methanoic acid ?
CO2 - the more reduced (more H) a carbon compound, the
more energy it can release upon oxidation
58
Oxidation- Reduction Reactions
- Alcohols can also be oxidized into other products
- Dr. Al K. Hall ? Mr. Al D. Hyde
- Equations middle of page 745
- Preparing aldehydes from a primary alcohol is a
problem, because they are then easily oxidized to
carboxylic acids
59
Oxidation- Reduction Reactions
- Benedicts test and Fehlings test are commonly
used for aldehyde detection Figure 23.19
p. 745
60
Section 23.4Polymerization
- OBJECTIVES
- Describe how addition polymers are formed.
61
Section 23.4Polymerization
- OBJECTIVES
- Describe how condensation polymers are formed.
62
Addition Polymers
- Polymers are giant molecules, not small like the
ones studied earlier in this chapter - examples are plastics
- Polymer- large molecule formed by the covalent
bonding of smaller molecules called monomers
63
Polymers from Monomers
64
Addition Polymers
- An addition polymer forms when unsaturated
monomers react to form a polymer - ethene will form polyethylene, shown on page 747
- polyethylene is easy to clean, chemically
resistant- milk bottles, plastic wrap,
refrigerator dishes
65
High Density Polyethylene
66
Addition Polymers
- Polypropylene is a stiffer polymer, used in
utensils and containers - Polystyrene is formed from styrene
(phenylethene), and is a poor heat conductor
(styrofoam Dow Chemical) - molded coffee cups and picnic coolers, insulates
homes - Polyvinyl chloride (PVC) used for pipes in
plumbing
67
Addition Polymers
- Polytetrafluoroethene (PTFE, or teflon DuPont)
is very resistant to heat and chemical corrosion - found on nonstick cookware coating on bearings
and bushings used in chemical reactors
68
Condensation Polymers
- Condensation polymers are formed by the
head-to-tail joining of monomer units - usually accompanied by the loss of water from the
reacting monomers, and forming water as a product
69
Condensation Polymers
- Ex polyethylene terephthalate (PET)
- Dacron ( DuPont), Fortrel ( Wellman),
Polyesters permanent press clothing, tire cords - Sheets of polyester called Mylar ( DuPont), used
as magnetic tape in tape recorders and computers,
as well as balloons - Nylon carpet, fishing line, hosiery
70
Condensation Polymers
- Examples
- aromatic rings form Nomex ( DuPont), which is a
poor electrical conductor makes parts for
electrical fixtures flame resistant clothing for
race car drivers flame resistant building
materials - Kevlar ( DuPont) strong and flame resistant
71
Plastic container code system.
72
What Do the Numbers Mean?
- 1 -- PETE (Polyethylene terephthalate)
- PET (or PETE) is used in the production of soft
drink bottles, peanut butter jars... - PET can be recycled into fiberfill for sleeping
bags, carpet fibers, rope, pillows...
73
What Do the Numbers Mean?
- 2 -- HDPE (High-density polyethylene)
- HDPE is found in milk jugs, butter tubs,
detergent bottles, motor oil bottles... - HDPE can be recycled into flower pots, trash
cans, traffic barrier cones, detergent bottles...
74
What Do the Numbers Mean?
- 3 -- V (Polyvinyl chloride)
- PVC is used in shampoo bottles, cooking oil
bottles, fast food service items... - PVC can be recycled into drainage and irrigation
pipes...
75
What Do the Numbers Mean?
- 4 -- LDPE (Low-density polyethylene)
- LDPE is found in grocery bags, bread bags, shrink
wrap, margarine tub tops... - LDPE can be recycled into new grocery bags...
76
What Do the Numbers Mean?
- 5 -- PP (Polypropylene)
- PP is used in most yogurt containers, straws,
pancake syrup bottles, bottle caps.... - PP can be recycled into plastic lumber, car
battery cases, manhole steps...
77
What Do the Numbers Mean?
- 6 -- PS (Polystyrene)
- PS is found in disposable hot cups, packaging
materials (peanuts), and meat trays... - PS can be recycled into plastic lumber, cassette
tape boxes, flower pots...
78
What Do the Numbers Mean?
- 7 -- Other
- This is usually a mixture of various plastics,
like squeeze ketchup bottles, "microwaveable"
dishes...
79
Timeline of Plastics
1862 First man-made plastic 1866 Celluloid
makes its debut 1891 Rayon is discovered 1907
Bakelite is invented 1913 Cellophane causes
the plastics craze
80
Timeline of Plastics
1926 PVC is invented 1933 Polyethylene is
discovered 1933 Saran makes its debut 1938
Teflon is discovered 1939 Nylon stockings hit
market 1957 Here comes velcro
81
End of Chapter 23