CHEMICAL EQUILIBRIUM - PowerPoint PPT Presentation
1 / 22
Title:
CHEMICAL EQUILIBRIUM
Description:
Thermodynamics A system, e.g. a mineral, is an isolated portion of the Universe. Internal Energy (E) of a mineral = Sum of energy stored in the bonding and in the ... – PowerPoint PPT presentation
Number of Views:142
Avg rating:3.0/5.0
Title: CHEMICAL EQUILIBRIUM
1
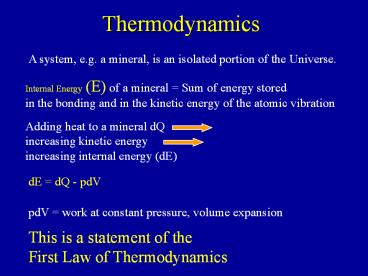
Thermodynamics
A system, e.g. a mineral, is an isolated portion
of the Universe.
Internal Energy (E) of a mineral Sum of energy
stored in the bonding and in the kinetic energy
of the atomic vibration
Adding heat to a mineral dQ increasing kinetic
energy increasing internal energy (dE)
dE dQ - pdV
pdV work at constant pressure, volume expansion
This is a statement of the First Law of
Thermodynamics
2
Thermodynamics
Added heat (Q) to a system versus its Entropy (S)
At constant temperature dQ/T dS Second law of
thermodynamics In any reversible process the
change in the Entropy of the system (dS) is equal
to the heat received by the system (dQ) divided
by the absolute temperature.
- Adding heat to ice increase in entropy through
- Vibration
- Breaking hydrogen bonds between (H2O) groups
- (Partial Melting)
3
Thermodynamics
Combinations of first and second law of
thermodynamics E Q PV, Q H at constant P H
E PV dQ TdS dE TdS - PdV G H TS G
E PV TS dG dE PdV VdP TdS SdT dG
TdS PdV PdV VdP TdS SdT
4
Free Energy vs. Temperature
- Slope of GLiq gt Gsol since
- Ssolid lt Sliquid
- A Solid more stable than liquid (low T)
- B Liquid more stable than solid (high T)
- Slope dG/dT -S
- Slope S lt Slope L
- Equilibrium at Teq
- GLiq GSol
dG VdP SdT
5
Free Energy vs. Pressure
- Slope of GLiq gt Gsol since
- Vsolid lt Vliquid
- A Solid more stable than liquid (high P)
- B Liquid more stable than solid (low P)
- Slope dG/dP V
- Slope S lt Slope L
- Equilibrium at Peq
- GLiq GSol
dG VdP SdT
6
Thermodynamics
dG VdP SdT In a phase diagram with the
environmental variables temperatures and
pressures we can relate the change of the free
energy of a phase A as dGA VAdP SAdT
and of the phase B dGB VBdP SBdT
7
Thermodynamics
At equilibrium dGB dGA or VBdP SBdT
VAdP SAdT (VA- VB)dP (SA
SB)dT dP/dT ? S/ ? V Clapeyron equation
8
Thermodynamics
- dG VdP SdT,
- at constant T dG VdP
- Ideal gas law V RT/P
- dG RT(dP/P)
- Integrating from the standard pressure P
- to a pressure P
- Gp G RT(lnP - lnP)
- (n GR)products S(nGR)reactants
RT(S(nlnP)products S(nlnP)reactants)
9
Thermodynamics
RT(S(nlnP)products S(nlnP)reactants) RT ln
K - DGR RT ln K R 1.987cal/deg mol T
298.15 K ln K 2.3025 log K DGR -1.364 log
K K 10-DGR/1.364
Note R 8.314 J/deg mol
10
The Equilibrium Constant
- For a one-component system, the Second Law of
Thermodynamics is - dG -SdT VdP
- but dG ndG ndµ
- where µ (?G/?n)
- At constant T and for simplicity an ideal gas,
- dG VdP
- From the gas law, V nRT/P
- Integrating,
- µ P
- n?µ? dµ nRT?P? dP
- µ - µº RT ln P/P?
- If P? is the standard state of the ideal gas at 1
atm pressure and T, P?1 - µ µº RT ln P
- (Note that P is unitless as it is a ratio as
defined above.)
11
The Equilibrium Constant
- For a multi-component gas mixture at equilibrium
- vj1Aj1 . . . vi-1Ai-1 viAi v1A1 v2A2
. . . vjAj - where v refers to stoichiometric coefficients and
A to the gas species. - The subscripts j1 to I refer to the reactants
and 1 to j refer to the - products.
12
The Equilibrium Constant
- µi µi RT ln Pi.
- where Pi P (ni /n) PXi
- ?Gr ?viµi
- ?vi (µiº RT ln Pi)
- ?viµiº ?viRT ln Pi
- ?G? RT ?ln Pivi
- ?G? RT ln K
- where
- P1v1P2v2 . . . Pj v j
- K Pj1vj1Pj2vj2 . . . Pivi the
equilibrium constant - (note that the value of K is dependent on the
quanitites used in the expression and the
standard states selected for them. In other
words, K is not a universal constant for a
reaction, but only for the conditions considered
in deriving it.)
13
Thermodynamics
DGR RT ln K ln K - DGR / RT DGR DHR
TDSR lnK -(DHR/T DSR)/ R dlnK/dT DHR /
RT2 dln K DHR/R (dT/T2) dln K DHR/R
(dT/T2)
14
TEMPERATURE DEPENDENCE OF THE EQUILIBRIUM CONSTANT
Vant Hoff Equation
15
EXAMPLE VANT HOFF PLOT
16
Application Solubility of silica
SiO2(am) 2H2O(l) ? H4SiO40 Gf(H4SiO40)
-312.66 kcal Gf(SiO2(am)) -203.33
kcal nGf(2H2O(l)) 2(-56.687) kcal DGR
-312.66 (-203.33 2(-56.687) 4.044 kcal K
10-2.96 DHR 3.47 kcal, endothermic
reaction Log KT -2.96 3.47x103(cal) /
(2.3025 x 1.987) x (1/T 1/298.15) Log KT
-2.96 758.4(1/T 0.00335)
17
Application Solubility of silica
Log KT -2.96 758.4(1/T 0.00335) With
increasing T, the term (1/T 0.00335) becomes
negative, the term 758.4(1/T 0.00335) becomes
positive and log KT gt -2.96 If the enthalpy
change is negative (exothermic), the last term
will be negative with increasing T and log KT lt
log K(298.15) Endothermic reaction K increases
with T Exothermic reactions K decreases with T
18
Application Solubility of silica
Endothermic reaction K increases with
T Exothermic reactions K decreases with
T Endothermic dissolution reaction higher
ratio of products versus reactants increasing
solubility with increasing T Exothermic
dissolution reactions lower ratio of products
versus reactants decreasing solubility with
increasing T
19
Second Example dissolution of feldspar
2KAlSi3O8 9H2O 2H ? Al2Si2O5(OH)4 2K
4H4SiO4 Equilibrium constant K at 25 and 100C???
Compound Gf (kcal) Hf (kcal)
KAlSi3O8 -894.9 -948.7
H2O -56.687 -68.315
Al2Si2O5(OH)4 -906.84 -983.5
K -67.70 -60.32
H4SiO4 -312.66 -348.3
20
DH R S (n Hf)products - S(n
Hf)reactants (-983.5 2(-60.32) 4(-348.3))
(2(-948.7) 9(-68.315)) -2497.34 -2512.235
14.895 kcal
Compound Gf (kcal) Hf (kcal)
KAlSi3O8 -894.9 -948.7
H2O -56.687 -68.315
Al2Si2O5(OH)4 -906.84 -983.5
K -67.70 -60.32
H4SiO4 -312.66 -348.3
21
- DGR 7.103 kcal
- DH R 14.895 kcal
- K 10-DGR/1.364 10-5.207
- Log K -5.207 at 298.15 K
- Log K at 373.15K?
R 1.987cal/deg mol
Log KT -5.207 3275.36(-0.000674) Log KT
-5.207 -2.208 Log KT -3.002
22
- DGR 7.103 kcal
- DH R 14.895 kcal
- K 10-DGR/1.364 10-5.207
- Log K -5.207 at 298.15 K
- Log K at 373.15K -3.002
- Endothermic dissolution reaction
- higher ratio of products versus reactants at
higher T - increasing solubility with increasing T