Overlap and Bonding - PowerPoint PPT Presentation
1 / 47
Title:
Overlap and Bonding
Description:
Title: Chapter 9 Molecular Geometries and Bonding Theories Author: John Bookstaver Last modified by: Software Manager Created Date: 3/7/2005 2:11:13 AM – PowerPoint PPT presentation
Number of Views:109
Avg rating:3.0/5.0
Title: Overlap and Bonding
1
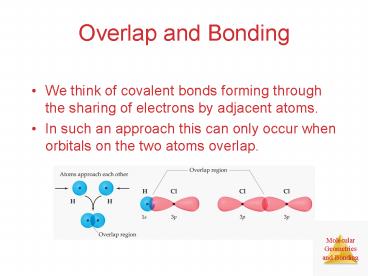
Overlap and Bonding
- We think of covalent bonds forming through the
sharing of electrons by adjacent atoms. - In such an approach this can only occur when
orbitals on the two atoms overlap.
2
Overlap and Bonding
- Increased overlap brings the electrons and nuclei
closer together while simultaneously decreasing
electron-electron repulsion. - However, if atoms get too close, the internuclear
repulsion greatly raises the energy.
3
- HYBRIDIZATION
- VALENCE BOND THEORY (s and p bonds)
- BOND ORDER
4
Hybrid Orbitals
- Its hard to imagine tetrahedral, trigonal
bipyramidal, and other geometries arising from
the atomic orbitals we recognize.
5
Hybrid Orbitals
- Atomic orbitals can mix or hybridize in order to
adopt an appropriate geometry for bonding. - Hybridization is determined by the electron
domain geometry. - sp Hybrid Orbitals
- Consider the BeF2 molecule (experimentally known
to exist)
6
Hybrid Orbitals
- Consider beryllium
- In its ground electronic state, it would not be
able to form bonds because it has no
singly-occupied orbitals.
7
Hybrid Orbitals
- But if it absorbs the small amount of energy
needed to promote an electron from the 2s to the
2p orbital, it can form two bonds.
8
Hybrid Orbitals
- Mixing the s and p orbitals yields two degenerate
orbitals that are hybrids of the two orbitals. - These sp hybrid orbitals have two lobes like a p
orbital. - One of the lobes is larger and more rounded as is
the s orbital.
9
Hybrid Orbitals (sp)
- These two degenerate orbitals would align
themselves 180? from each other. - This is consistent with the observed geometry of
beryllium compounds linear.
10
Hybrid Orbitals
- With hybrid orbitals the orbital diagram for
beryllium would look like this. - The sp orbitals are higher in energy than the 1s
orbital but lower than the 2p.
11
(No Transcript)
12
- Since only one of the Be 2p orbitals has been
used in hybridization, there are two unhybridized
p orbitals remaining on Be.
13
- sp2 Hybrid Orbitals
- Important when we mix n atomic orbitals we must
get n hybrid orbitals. - sp2 hybrid orbitals are formed with one s and two
p orbitals. (Therefore, there is one
unhybridized p orbital remaining.) - The large lobes of sp2 hybrids lie in a trigonal
plane. - All molecules with trigonal planar electron pair
geometries have sp2 orbitals on the central atom.
14
Hybrid Orbitals (sp2)
- For boron
15
Hybrid Orbitals
- three degenerate sp2 orbitals.
16
Hybrid Orbitals
- With carbon we get
17
Hybrid Orbitals
- four degenerate
- sp3 orbitals.
18
- sp2 and sp3 Hybrid Orbitals
- sp3 Hybrid orbitals are formed from one s and
three p orbitals. Therefore, there are four
large lobes. - Each lobe points towards the vertex of a
tetrahedron. - The angle between the large lobs is 109.5?.
- All molecules with tetrahedral electron pair
geometries are sp3 hybridized.
19
- Hybridization Involving d Orbitals
- Since there are only three p-orbitals, trigonal
bipyramidal and octahedral electron domain
geometries must involve d-orbitals. - Trigonal bipyramidal electron domain geometries
require sp3d hybridization. - Octahedral electron domain geometries require
sp3d2 hybridization. - Note the electron domain geometry from VSEPR
theory determines the hybridization.
20
Sp3d and sp3d2 Hybrid Orbitals
- For geometries involving expanded octets on the
central atom, we must use d orbitals in our
hybrids.
21
Hybrid Orbitals
- This leads to five degenerate sp3d orbitals
- or six degenerate sp3d2 orbitals.
22
Hybrid Orbitals
- Summary
- Draw the Lewis structure.
- Determine the electron domain geometry with
VSEPR. - Specify the hybrid orbitals required for the
electron pairs based on the electron domain
geometry.
23
(No Transcript)
24
(No Transcript)
25
Hybrid Orbitals
- Once you know the electron-domain geometry, you
know the hybridization state of the atom.
26
- Examples Determine the hybridization on the
central atom of each - NCl3
- CO2
- H2O
- SF4
- BF3
- XeF4
27
- Examples Determine the hybridization on the
central atom of each - NCl3 sp3
- CO2 sp
- H2O sp3
- SF4 sp3d
- BF3 sp2
- XeF4 sp3d2
28
- Examples Determine the hybridization on EACH
atom
29
- Examples Determine the hybridization on EACH
atom
sp2 sp
sp3
sp3
sp2
30
Valence Bond Theory
- Hybridization is a major player in this approach
to bonding. - There are two ways orbitals can overlap to form
bonds between atoms.
31
Sigma (?) Bonds
- Sigma bonds are characterized by
- Head-to-head overlap.
- Cylindrical symmetry of electron density about
the internuclear axis.
32
Pi (?) Bonds
- Pi bonds are characterized by
- Side-to-side overlap.
- Electron density above and below the internuclear
axis.
33
Single Bonds
- Single bonds are always ? bonds, because ?
overlap is greater, resulting in a stronger bond
and more energy lowering.
34
Multiple Bonds
- In a multiple bond one of the bonds is a ? bond
and the rest are ? bonds.
35
Multiple Bonds
- In a molecule like formaldehyde (shown at left)
an sp2 orbital on carbon overlaps in ? fashion
with the corresponding orbital on the oxygen. - The unhybridized p orbitals overlap in ? fashion.
36
Multiple Bonds
- In triple bonds, as in acetylene, two sp orbitals
form a ? bond between the carbons, and two pairs
of p orbitals overlap in ? fashion to form the
two ? bonds.
37
Delocalized Electrons Resonance
- When writing Lewis structures for species like
the nitrate ion, we draw resonance structures to
more accurately reflect the structure of the
molecule or ion.
38
Delocalized Electrons Resonance
- In reality, each of the four atoms in the nitrate
ion has a p orbital. - The p orbitals on all three oxygens overlap with
the p orbital on the central nitrogen.
39
Delocalized Electrons Resonance
- This means the ? electrons are not localized
between the nitrogen and one of the oxygens, but
rather are delocalized throughout the ion.
40
Resonance
- The organic molecule benzene has six ? bonds and
a p orbital on each carbon atom.
41
Resonance
- In reality the ? electrons in benzene are not
localized, but delocalized. - The even distribution of the ?? electrons in
benzene makes the molecule unusually stable.
42
- General Conclusions
- Every two atoms share at least 2 electrons.
- Two electrons between atoms on the same axis as
the nuclei are ? bonds. - ?-Bonds are always localized.
- If two atoms share more than one pair of
electrons, the second and third pair form
?-bonds. - When resonance structures are possible,
delocalization is also possible.
43
- Bond Order
- Bond Order total number of covalent bonds
between 2 atoms - Bond order 1 for single bond.
- Bond order 2 for double bond.
- Bond order 3 for triple bond.
- If there is resonance then divide the bond order
by the number of atoms that share the resonance
structure. - Fractional bond orders are possible (with
resonance)
44
- Examples Draw Lewis Structures (including
resonance). Determine the total number of s and
p bonds in the molecule, and the bond order of
each bond - O3
- SO3
- CO2
45
- Examples Draw Lewis Structures (including
resonance). Determine the total number of s and
p bonds in the molecule, and the bond order of
each bond - O3
- SO3
- CO2
2s and 1p Each bond 1.5
3s and 1p each bond 1.33
2s and 2p each bond 2
46
- Electron Configurations and Molecular Properties
- Two types of magnetic behavior
- paramagnetism (unpaired electrons in atom or
molecule) strong attraction between magnetic
field and molecule - diamagnetism (no unpaired electrons in atom or
molecule) weak repulsion between magnetic field
and molecule. - Magnetic behavior is detected by determining the
mass of a sample in the presence and absence of
magnetic field
47
Second-Row Diatomic Molecules
- Electron Configurations and Molecular Properties
- large increase in mass indicates paramagnetism,
- small decrease in mass indicates diamagnetism.