Relationship between mass, moles and molecules in a compound - PowerPoint PPT Presentation
1 / 15
Title:
Relationship between mass, moles and molecules in a compound
Description:
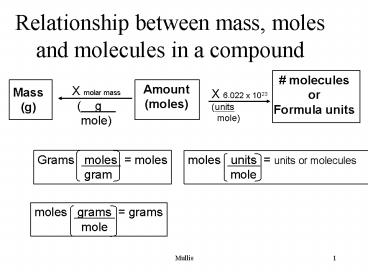
Relationship between mass, moles and molecules in a compound Amount (moles) # molecules or Formula units X molar mass (__g__ mole) Mass (g) X 6.022 x 1023 – PowerPoint PPT presentation
Number of Views:123
Avg rating:3.0/5.0
Title: Relationship between mass, moles and molecules in a compound
1
Relationship between mass, moles and molecules in
a compound
X molar mass (__g__ mole)
Mass (g)
X 6.022 x 1023 (units mole)
Grams moles moles gram
moles units units or molecules
mole
moles grams grams mole
2
Molar mass
- Molar mass of a substance mass in grams of one
mole of the substance. - A compounds molar mass is NUMERICALLY equal to
its formula mass. - 2 mol H x 1.01 g H 2.02 g H
- 1 mol H
- 1 mol O x 16.00 g O 16.00 g O
- 1 mol O molar mass H2O
- 18.02 g/mol
- Formula mass H2O 18.02 amu
- Molar mass H2O 18.02 g/mol
3
Molar Mass Example
- What is the molar mass of K2SO4?
- 2 mol K x 39.10 g K 78.20 g K
- 1 mol K
- 1 mol S x 32.10 g S 32.07 g S
- 1 mol S
- 4 mol O x 16.00 g O 64.00 g O
- 1 mol O
- molar mass K2SO4
- 174.27 g/mol
- How many moles of each element are present in
this compound? - 2 mol K, 1 mol S, 4 mol O
4
- What is the molar mass of C6H12O6?
- 6 mol C x 12.01 g C 72.06 g C
- 1 mol C
- 12 mol H x 1.01 g H 12.12 g H
- 1 mol H
- 6 mol O x 16.00 g O 96.00 g O
- 1 mol O
- molar mass C6H12O6
- 180.18 g/mol
- How many moles of each element are present in
this compound? - 6 mol C, 12 mol H, 6 mol O
5
Converting to grams from moles
- How many moles of glucose are in 4.15x10-3 g
C6H12O6? - 4.15x10-3 g x 1 mol C6H12O6 2.30 x 10-5 mol
C6H12O6 - 180.18 g
- How many molecules of glucose are in 4.15x10-3 g
C6H12O6? - 2.30 x 10-5 mol C6H12O6 x 6.022 x 10 23
molecules - 1 mol
- (2.30 x 6.022)(10(-523)) 13.90 x 10 18
molecules - 1.39 x 10 19 molecules
6
- What is the mass in grams of 6.25 moles copper
(II) nitrate? - Cu 2 NO3 - formula is Cu(NO3)2
- Find molar mass of Cu(NO3)2 first.
- 1 mol Cu x 63.55 g Cu 63.55 g Cu
- 1 mol Cu
- 2 mol N x 14.01 g N 28.02 g N
- 1 mol N
- 6 mol O x 16.00 g O 96.00 g O
- 1 mol O
- molar mass Cu(NO3)2 187.57 g/mol
- Now find mass in grams of 6.25 moles
- 6.25 moles x 187.57 g 1172 g Ans. 1170 g
Cu(NO3)2 - 1 mol
7
Atoms and Ions Within Compounds
- How many carbon atoms are in one mole of C2H6?
- 1 mole C2H6 2 moles C 6.022 x 1023 atoms
1.204 x 1024 atoms - 1 mole C2H6 1 mole C
- How many MOLES of carbon atoms are in one mole of
C2H6? - 1 mole C2H6 2 moles C 2 moles C atoms
- 1 mole C2H6
- How many moles of hydroxide ions are in one mole
of calcium hydroxide? How many moles of Ca2? - 1 mole Ca(OH)2 2 moles OH-- 2 moles
hydroxide ions - 1 mole Ca(OH)2
- 1 mole Ca(OH)2 1 mole Ca2 1 mole calcium
ions - 1 mole Ca(OH)2
8
Percentage Composition
- Composition is the by mass of each element
in a compound. - Find the percentage composition of sodium
chloride. - Na Cl - formula is NaCl
- 1 mol Na x 22.99 g Na 22.99 g Na
- 1 mol Na
- 1 mol Cl x 35.45 g Cl 35.45 g Cl
- 1 mol Cl molar mass NaCl 58.44
g/mol - 22.99 g Na x 100 39.34 Na
- 58.44 g NaCl
- 35.45 g Cl x 100 60.66 Cl
- 58.44 g NaCl
9
- Find the percentage composition of sodium
nitrate. - Na NO3 - formula is NaNO3
- 1 mol Na x 22.99 g Na 22.99 g Na
- 1 mol Na
- 1 mol N x 14.01 g N 14.01 g N
- 1 mol N
- 3 mol O x 16.00 g O 48.00 g O
- 1 mol O molar mass NaNO3
85.00 g/mol - 22.99 g Na x 100 27.05 Na
- 85.00 g NaNO3
- 14.01 g N x 100 16.48 N
- 85.00 g NaNO3
- 48.00 g O x 100 56.47 O
- 85.00 g NaNO3
10
Percentage Composition
- Why mass instead of moles?
- Isnt 2/3 of the water molecule hydrogen?
- Moles indicate the amounts of each atom needed to
make the molecule stable from an electron
standpoint. - 2.02 g H x 100 11.21 H
- 18.02 g H2O
O
H
H
11
Empirical Formula
- Use composition to convert to original formula
- Assume 100 g sample, so grams
- Convert grams to moles for each element
- Divide the number of moles for each element by
the smallest number of moles - The result for each type of element is its
subscript in the empirical formula. - The order of elements is usually
- Organics C,H,O,N
- Inorganics Metal, nonmetal, oxygen
- Keep 4 decimal places when dividing numbers. If
the result has a decimal between .2 and .8, may
need to multiply all numbers by the number needed
to get a whole number. - Ex 3.5 should be multiplied by 2 to get 7.
Then multiply all other elements by the same
number.
12
Empirical Formula, cont.
13
Example Empirical and Molecular formula
- What is the empirical formula of a compound with
54.82 C, 5.624 H, 32.45 O, 7.104 N? - 54.82 g C 1 mole C 4.568 mole C / .5074 9 C
- 12 g C
- 5.624 g H 1 mole H 5.624 mole H / .5074 11 H
- 1 g H C9H11O4N
- 32.45 g O 1 mole O 2.028 mole O / .5074 4 O
- 16 g O
- 7.104 g N 1 mole N 0.5074 mole N / .5074 1 N
- 14 g N
- If a compound has this same composition but its
molecular weight is 394 g/mol, what is its
molecular formula? - MW. C9H11O4N 197 g/mol
- 394/197 2 so molecular formula is C18H22O8N2
14
Oxidation Numbers
- Used to indicate the general distribution of
electrons among the bonded atoms in molecular
compounds or polyatomic ions. - Analogous to charges in ionic compounds.
- An oxidation number is assigned to each element.
- Assign the ones you know 1st.
- Find the others based on the numbers it takes to
make the charge equal to the charge of the ion or
compound. (A compound has a charge of zero.)
15
Oxidation Numbers Rules
- Pure element 0
- F -1
- O -2 (except in peroxides and bonds with
halogens) - H 1 (except in bonds with metals)
- The more electronegative element same (-)
charge as its anion - The less electonegative element same () charge
as its cation - The sum of a compound or polyatomic ions
oxidation numbers is equal to its charge.