Intro to light - Flame Test - PowerPoint PPT Presentation
1 / 25
Title:
Intro to light - Flame Test
Description:
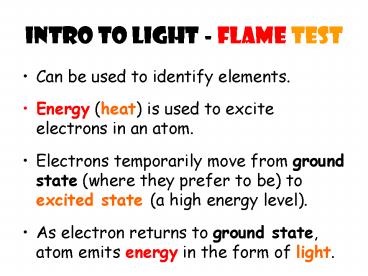
Intro to light - Flame Test Can be used to identify elements. Energy (heat) is used to excite electrons in an atom. Electrons temporarily move from ground state ... – PowerPoint PPT presentation
Number of Views:218
Avg rating:3.0/5.0
Title: Intro to light - Flame Test
1
Intro to light - Flame Test
- Can be used to identify elements.
- Energy (heat) is used to excite electrons in an
atom. - Electrons temporarily move from ground state
(where they prefer to be) to excited state (a
high energy level). - As electron returns to ground state, atom emits
energy in the form of light.
2
Gulp!!!
Burp!!
Photon
Shake!!
3
Electromagnetic Radiation(light)
- Produced by changing electric and magnetic fields
- Can be thought of as both a particle and a wave
(wave-particle duality) - Does NOT require a medium
- Transverse
4
HOMEWORK
Mechanical waves
Electromagnetic waves
Read pgs. 500, 532, 533
5
Electromagnetic Waves
- Homework Assignment
- Read pg. 500, 532, 533
- Make a double bubble map to compare and contrast
electromagnetic waves to mechanical waves.
6
Light as a Wave
- When we treat light as a wave, it has the
properties of any other wave - Wavelength
- Frequency
- Speed
- These properties are related just like they are
with mechanical waves. - What is the formula?
7
Electromagnetic RADiation
- The Sun is the source of EM radiation on Earth
- Speed remains the same all EM waves travel at
the speed of light - 3.00 x 108 m/s
8
Practice Problems
- What is the wavelength of an electromagnetic wave
that has a frequency of 4.03x1014 Hz? - 2. What is the frequency of an electromagnetic
wave that has a wavelength of 3 m?
9
Electromagnetic Spectrum
- When we think of light as a wave, we discover
that electromagnetic radiation (light) can come
in many forms. These forms depend on the
wavelength and frequency of the light. - Electromagnetic waves of different
frequency/wavelength fall onto different places
on the electromagnetic spectrum.
10
Electromagnetic Spectrum
Left to Right wavelength decreases, frequency
increases
11
Electromagnetic Spectrum
12
Radio Waves
- Used in radio and television transmission.
13
microWaves
- Used in microwave appliances
- Radar waves are also
- a form of microwaves
14
Infared waves
- Show the amount of thermal energy (heat) a
particular object has. - Heat or Night Vision
15
Visible Light waves
- Waves our eyes are capable of seeing
R O Y G B I V
16
Ultraviolet Waves
- First potentially harmful form of EM radiation
- Overexposure can lead to skin cancer
17
X-Rays
- Used in the medical field to look inside the
human body
18
Gamma Rays
- Highest energy form of EM radiation
- Hardest radiation to be protected from
- Used in treatment of cancer (radiationtreatment)
19
What is giving off radiation?
- By studying and displaying the em radiation given
off by stars, scientists can determine the
chemical composition of those stars among other
things.
20
Spectrum/Spectra
- Spectrum ? to made a display out of something
- In astronomy, we display radiation using
spectroscopes (bend light to see various
wavelengths being emitted) - Each element produces a set of characteristic
emission lines
21
- From spectral lines astronomers can determine not
only the element, but the temperature and density
of that element in the star. The spectral line
also can tell us about any magnetic field of the
star. The width of the line can tell us how fast
the material is moving. We can learn about winds
in stars from this. If the lines shift back and
forth we can learn that the star may be orbiting
another star. We can estimate the mass and size
of the star from this. If the lines grow and fade
in strength we can learn about the physical
changes in the star. Spectral information can
also tell us about material around stars. This
material may be falling onto the star from a
doughnut-shaped disk around the star called an
accretion disk. These disks often form around a
neutron star or black hole. The light from the
stuff between the stars allows astronomers to
study the interstellar medium (ISM). This tells
us what type of stuff fills the space between the
stars. Space is not empty! There is lots of gas
and dust between the stars. Spectroscopy is one
of the fundamental tools which scientists use to
study the Universe.
22
Spectra
- Continuous
- A luminous solid or liquid emits a continuous
spectrum of all wavelengths. It has no lines in
it.
- Discrete
Emission When hot gas is emitted its own light
Absorbtion When light from a brigher source is
shone through it
23
(No Transcript)
24
Signatures of elements
25
Sun Spectrum