Basic chemistry for understanding biology - PowerPoint PPT Presentation
1 / 46
Title: Basic chemistry for understanding biology
1
(No Transcript)
2
CHAPTER 3
- Physical and Chemical Properties of Water
3
The properties and biological importance of water
- Life on Earth evolved in the water, and all life
still depends on water. - 80 of living organism mass.95 of a jellyfishs
- Almost all the chemical reactions of life take
place in aqueous solution - Water formula H2O
- 2 Hydrogen atoms
- 1 Oxygen atom
4
Waters atoms
5
Properties of Water
Covalent bonding vs. Hydrogen bonding
Covalent Bond
Since Oxygen needs 2 electrons, 2 hydrogens will
bond to Oxygen. Each Hydrogen also needs 1
electron, so together, they all share 2 electrons
or covalent bonds.
6
Water H2O
- Water molecules are charged, with the oxygen atom
being slightly negative, and the hydrogen atoms
being slightly positive . These opposite charges
attract each other, forming hydrogen bonds.
7
States of Matter Water molecules
Water turns to ice at 0 degrees Celsius, and
vapor at 100 degrees Celsius
8
Properties of Water - Density
- Density mass/volume
- Water is unique in that the solid state (ice) is
less dense than the liquid state, so ice floats
on water. - Water molecules get further apart as it freezes,
causing it to EXPAND The decrease in density
causes ice to float. - This allows aquatic ecosystems to exist even in
sub-zero temperatures.
9
(No Transcript)
10
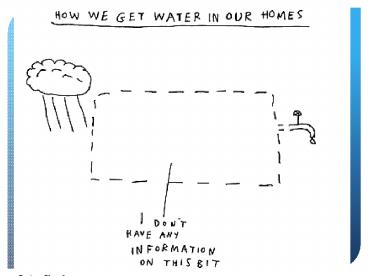
Water as a solvent
- Solvent A liquid, solid, or gas that dissolves
another liquid, solid, or gas called solutes. - When many salts dissolve in water they ionize
into positive and negative ions - ex. NaCl Na Cl-
- Ions- electrically charged particles, water
molecules are very attracted to them
11
(No Transcript)
12
Properties of Water - Cohesion
- Cohesion stick together
- Water molecules "stick together" due to their
hydrogen bonds - Surface tension is caused by cohesion
13
Salinity
- Six ions compose over 99 of dissolved solids in
water - Sodium and Chloride make up 85 of that (explains
why ocean water tastes salty) - Salinitytotal amount of salt dissolved in
seawater - If 35 grams of salt remain when 1,000 grams of
seawater evaporates salinity 35 parts per
thousand (ppt or psu) - This is the avg of open ocean
14
Salinity of ocean water varies
- Inflow from river, evaporation rates, melting of
ice, precipitation, ocean currents - SALTIEST Red Sea/Persian Gulf and North Atlantic
(40ppt)
15
Rule of Constant Proportions
- The relative amounts of various ions in seawater
always stay the same, regardless of its salinity
16
Salinity Temperature? Density
- Temperature affect waters density
- As temps ? Density / Temps ? Density
- Salinity affects waters density
- As salinity ? Density / Salinity ?
Density - Soooo it gets denser as it gets saltier, colder
or both
17
Buoyancy
- Upward force that keeps things afloat
- Floats ()buoyant, Sinks (-) buoyant, Hovers
neutral
18
- The amount of pressure we experience at sea level
is one atmosphere (1 atm) or 14.7 psi
(pounds/square inch) - In water, pressure increases 1 atm every 10 m of
depth - Scuba divers and marine organisms have the weight
of the water column on them
19
http//www.youtube.com/watch?vYU2PSHeFSlA
20
Pressure and Density
- Water is incompressible (you cannot decrease its
volume with added pressure) negligible - Gases ARE compressible due to Boyles Law
PVconstant - If P , then V and vice versa
21
- IN GASESmolecules are further apart, more room
to squeeze together liquids and solids dont
compress - Density of air INCREASES when under pressure
- Examples of compressed air (gas)
3,000psi
40psi
65psi
22
Scuba diver tank
- Made of thick aluminum
- Rounded edges
- Air is put in tank w/ compressor
- 50 ft3 squeezed in, requires 3,000psi OMG!
1 ft3
23
Cartesian Diver
- Things you will need
24
- Bring supplies Tomorrow!!
- This will be your chance to make mistakes
- You will need a working, functional cartesian
diver that I will test out individually
25
(No Transcript)
26
(No Transcript)
27
Same process takes place in fish swim bladder.
Muscles compress/decompress the air in bladder to
control how much it floats
28
(No Transcript)
29
The Bends
- N gas dissolves into bloodstream at high
pressures. - If diver surfaces too quickly to lower pressure,
the gases come out as bubbles in your tissue - Extreme pain/tissue damage
- Decompression chamber to dissolve gas into blood
again, then reduce pressure slowly
30
Oxygen Solubility
- Oxygen is MORE soluble (dissolved) into colder
water - Plankton? fish more abundant in
cooler/deeper/nutrient-rich water - Oxygen is LESS soluble in warmer water
- Plankton? fish less abundant in nutrient-poor
warm water
31
Properties of Water - pH
- Water itself is partly ionized
- H2O H OH-
- pH Scale 1-14
- Acidic pH 1-6
- Acid release H ions
- Neutral pH 7
- Basic pH 8-14
- Bases accept H ions
- Many biochemical reactions are sensitive to pH
32
(No Transcript)
33
Transparency
- Allows sunlight to penetrate water to
photosynthetic organisms - Affected by material suspended or dissolved in
water - Secchi disk
34
Ocean Blue
- Water is more transparent to blue light
wavelength - Other colors of spectrum get absorbed at shorter
depths while blue can penetrate further down - Deep ocean organisms colored red, orange, purple.
35
What direction does water go down the toilet when
you flush??
36
Coriolis Effect
- Earth is round/rotating. Anything moving over
surface tends to get deflected - Winds and ocean currents bend to right in N.
Hemisphere and left in S. Hemisphere
37
In ONE word, what makes wind?
- SUN!!!!!!!!!
38
Wind
- Warm air at the equator rises (less dense)
- Adjacent air gets sucked in to replace rising
air.creating trade winds (come in at angle due
to Coriolis effect) - Steadiest winds on earth
- Westerlies and polar easterlies run opposite to
trade (vary)
39
Currents
- Wind hitting water creates surface currents that
move at 45 angle (due to Coriolis) - Top layer pushes water below that deflects at
different angle (Ekman spiral)
EKMAN TRANSPORT
40
Gyres
- Currents combine into a huge, circular systems
called gyres - Gyres warm the poles, cool the tropics and
regulate the earths climate
41
Three layer water column
- Surface (mixed layer)
- Thermocline layer (Intermediate)
- Deep layer (very cold)
42
Overturn
- If the surface water gets cold enough (winter),
it becomes denser and sinks. This is downwelling. - Displaces deeper water below, deep water rises
(upwelling)
Sometimes wind can cause this effect in lakes
43
The Great Ocean Conveyor
- Constantly replenishes ocean depths with oxygen
- Regulates Earths climate
44
- Waves travel through water they do not take the
water with them. - Bob up and down
- As a wave arrives it lifts water particles. These
travel forward, then down and back so that each
particle completes a circle. - Circling movements near the surface set off
smaller movements below them.
45
Tides
- Rise and fall of sea surface
- Caused by gravitational pull of moon and sun and
centrifugal force from rotation of Earth, moon
and sun - Spring tide (surge up like a spring of water)
- Neap tide (tidal range is small)
46
(No Transcript)


















![Welcome to CHEM BIO 3OA3! Bio-organic Chemistry [OLD CHEM 3FF3] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/P1258891208FGTDt.th0.jpg?_=20140110098)











