pH Scale - PowerPoint PPT Presentation
1 / 17
Title:
pH Scale
Description:
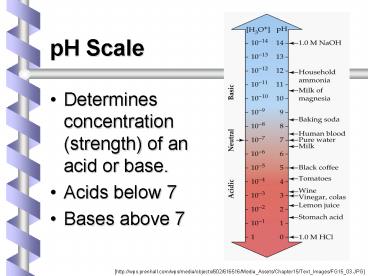
pH Scale Determines concentration (strength) of an acid or base. Acids below 7 Bases above 7 [http://wps.prenhall.com/wps/media/objects/602/616516/Media_Assets ... – PowerPoint PPT presentation
Number of Views:349
Avg rating:3.0/5.0
Title: pH Scale
1
pH Scale
- Determines concentration (strength) of an acid or
base. - Acids below 7
- Bases above 7
http//wps.prenhall.com/wps/media/objects/602/616
516/Media_Assets/Chapter15/Text_Images/FG15_03.JPG
2
pH Scale
- Indicators show the pH level in the lab.
http//wps.prenhall.com/wps/media/objects/602/616
516/Media_Assets/Chapter15/Text_Images/FG15_05.JPG
3
Click for indicator demo
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH15/FG15_
13.JPG
4
pH Scale
- pH is based on the concentration of the hydronium
ion.
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH15/FG15_
00-02.JPG
5
pH Scale
pH is not a linear scale!!
pH -logH3O
http//wps.prenhall.com/wps/media/objects/476/488
316/Instructor_Resources/Chapter_14/FG14_15.JPG
6
(No Transcript)
7
pH Scale
- Example If the concentration of hydronium ions
is 3.0 x 10-4 M, what is the pH? - pH -log3.0 x 10-4
- pH 3.5
8
pH Scale
- What about changing pH toH3O
- H3O10-pH
- Example If the pH of a base is 12.5, what is
H3O?
9
pH Scale
- H3O 10-12.5
- 3.16 x 10-13 M
- But waitdoesnt a base give off OH-???
10
Water as an Acid Base
http//wps.prenhall.com/wps/media/objects/476/488
316/Instructor_Resources/Chapter_14/FG14_14-01un.J
PG
11
Water as an Acid Base
- Keq H3OOH-
- Water is neutral so pH 7.
http//wps.prenhall.com/wps/media/objects/476/488
316/Instructor_Resources/Chapter_14/FG14_14-02un.J
PG
12
Water as an Acid Base
- H3O 1 x 10-7
- Also, H3O and OH- are equal since water is
neutral
http//wps.prenhall.com/wps/media/objects/476/488
316/Instructor_Resources/Chapter_14/FG14_14-02un.J
PG
13
Water as an Acid Base
- 1 x 10-14 H3OOH-
- This is known as Kw.
- (Equilibrium Water Constant)
http//wps.prenhall.com/wps/media/objects/476/488
316/Instructor_Resources/Chapter_14/FG14_14-02un.J
PG
14
pOH scale
- When NH3 is dissolved in water, it gives off the
Hydroxide Ion (OH-)
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH15/FG15_
00-04.JPG
15
pOH Scale
- pOH scale is reversed
- (0-7 are bases, 7-14 acids)
- pOH -logOH-
- OH-10-pOH
16
pH pOH
- pH pOH 14
- Example
- If OH- for a base is 2.00 x 10-2 M, what is
the pH?
17
pH pOH
- pOH -log2.00 x 10-2
- pOH 1.70
- pH 1.70 14
- pH 12.3