Electronic transitions - PowerPoint PPT Presentation
1 / 51
Title: Electronic transitions
1
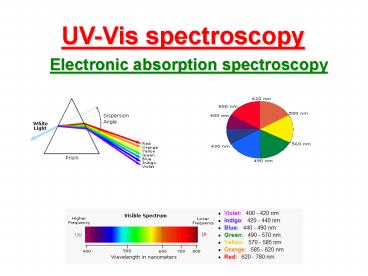
UV-Vis spectroscopy
Electronic absorption spectroscopy
2
spectroscopy
- The interactions of radiation and matter are
the subject of the science called spectroscopy.
3
Properties of Electromagnetic Radiation
- Electromagnetic radiation is a form of energy
that is transmitted through space at enormous
velocities. - Electromagnetic radiation have properties of
wavelength, frequency, velocity, and amplitude. - In contrast to sound waves, light requires no
supporting medium for its transmission thus, it
readily passes through a vacuum.
4
ELECTROMAGNETIC SPECTRUM
5
Absorption and Emission
Absorption
Emission
Absorption A transition from a lower level to a
higher level with transfer of energy from the
radiation field to an absorber, atom, molecule,
or solid. Emission A transition from a higher
level to a lower level with transfer of energy
from the emitter to the radiation field.
6
UV and Visible Spectroscopy
- Ultraviolet Spectroscopy involves the
measurement of absorption of light in the visible
and ultraviolet regions (visible region 400-800
nmUV region 200-400 nm) by the substances under
investigation. - Since the absorption of light involves the
transition from one electronic energy level to
another with in a molecule, UV spectroscopy is
also known as electronic spectroscopy.
7
PRINCIPLE OF UV SPECTROSCOPY
- Absorption of visible and ultraviolet light
produces changes in the electronic states of
molecules associated with the excitation of an
electron from a lower to a higher energy level. - But it must be noted that each el electronic
level in a molecule is associated with a no. of
vibrational sub-levels and each vibrational
energy level in turn is associated with a no. of
rotational sub-levels.
8
Appearance of broad bands and not sharp peaks in
the spectrum.
- Due to the mixing of vibrational and rotational
changes with electronic changes in the molecules,
there will be a large no. of possible transitions
requiring only slightly different energies. - As a result the absorption spectrum contains a
large no. of lines which are too close together
to be distinguished separately and are recorded
in the form of broad bands in the spectrum
obtained.
9
Electronic excitation from the ground state to
the excited state accompanied By changes in
vibrational and rotational sub-levels.
Here E0Ground state
EExcited state.
10
Beer-Lamberts Law
- B eer Law When a monochromatic light is
passed through a substance dispersed in a
non-absorbing solvent, the absorption of light is
directly proportional to the molar concentration
of the substance. - Lambert s Law When a of monochromatic light is
passed through a substance dispersed in a
non-absorbing solvent, the absorption of light is
directly proportional to the path length of the
sample. - B eer- Lambert s Law B y combining above two
Laws," The absorption of light by a substance at
a particular wavelength is directly proportional
to the no. of molecules of the substance in the
path of light.
11
Thus log I0/I a c (Beer's
Law) log I0/I a l
(Lambert'Law)
log I0/I a cl (Beer Lamberts
Law)
log I0/I cl log
I0/I Optical Density or absorbance (A)
Therefore,
(A)log I0/I cl
Where- A Absorbance (optical density) I0
Intensity of light on the sample cell I
Intensity of light leaving the sample cell c
molar concentration of solute l length of
sample cell (cm)
12
Limitation of Beer Lamberts Law
- High concentrations
- Solute and solvent form complexes
- Thermal equilibrium exist between the ground
state and the excited state - Fluorescent compounds are present in solution
13
Components of instrumentation
- 1) Sources Argon, Xenon, Deuterium, or Tungsten
lamps - Deuterium Lamps-a truly continuous spectrum in
the ultraviolet region is produced by electrical
excitation of deuterium at low pressure.
(160nm375nm) - Tungsten Filament Lamps-the most common source of
visible and near infrared radiation. - 2) Sample Containers Quartz, Borosilicate,
Plastic.
14
3) Monochromators Quartz prisms and all
gratings, Used as a filter the monochromator
will select a narrow portion of the spectrum (the
band pass) of a given source Used in analysis
the monochromator will sequentially select for
the detector to record the different components
(spectrum) of any source or sample emitting
light. 4) Detectors which continuously measures
the intensity ratio of the beams transmitted
through the sample and the solvent respectively.
15
Instrumentation
16
General Instrument Designs Double Beam Space
resolved
17
Presentation of the spectrum
p?p
18
Origin of electronic spectra
- Absorptions of UV-vis photons by molecule results
in electronic excitation of molecule with
chromophore. - The electronic transition involves promotion of
electron from a electronic ground state to higher
energy state, usually from a molecular orbital
called HOMO to LUMO.
19
UV-Vis light causes electrons in lower energy
molecular orbitals to be promoted to higher
energy molecular orbitals. HOMO LUMO
20
Electronic transitions
- There are following types of electronic
transition takes place in UV and visible region - s s Transitions
- n s Transitions
- n p transitions
- p p transitions
21
Vacuum UV or Far UV (? lt190 nm )
(Important in organic chemist)
UV/VIS
22
(No Transcript)
23
Electronic transition
- s s Transitions Transitions in which a s
bonding electron is excited to an antibonding s
orbital are called s s transitions. These
transitions are shown by only saturated
hydrocarbons. - For example, methane (which has only C-H bonds,
and can only undergo s s transitions) shows an
absorbance maximum at 125 nm. Absorption maxima
due to s s transitions - These wavelengths are lesser than 200 nm and
fall in the vacuum UV region.
24
n s Transitions
- n s Transitions Saturated compounds containing
atoms with lone pairs (non-bonding electrons) are
capable of n s transitions. - These transitions usually need less energy than s
s transitions. - They can be initiated by light whose wavelength
is in the range 150 - 250 nm. - The number of organic functional groups with n
s peaks in the UV region is small. - E.g., CH3Cl.
25
n p Transitions
- n p transitions These are the transitions in
which an electron in a non bonding atomic
orbital is promoted to an antibonding p orbital. - Compounds having double bonds between
heteroatoms, e.g., CO, CS, and NO. - For e.g. ,the gtCO group of saturated aldehydes
or ketones exhibit an absorption of low intensity
at about 285 nm. - These transitions require only small amounts of
energy and takes place with in the range of
ordinary UV spectrophotometer. - These are generally forbidden transitions.
26
p p Transitions
- p p transitions These are the transitions in
which an electron in a p electron is promoted to
an antibonding p orbital. - These transitions require relatively higher
amount of energy than n p transitions. - For e.g. ,the gtCO group of saturated aldehydes
or ketones exhibit an absorption of high
intensity at about 180 nm.
27
Selection Rules of electronic transition
- Electronic transitions may be classified as
intense or weak according to the magnitude of
emax that corresponds to allowed or forbidden
transition - 1)Allowed transitions These are transitions have
emax 104 or more and probability of their
occurrence is very high. These are generally due
to p p transition - For e.g., p p transition in 1,3-butadiene
which shows absorption at 217 nm and emax 20900
represents an allowed transition.
28
Forbidden Transition
- These are usually related to n p transition.
- These are the transitions for which emax is
generally less than 104 . - For e.g., n p transition of a saturated
aldehydes or ketones exhibit a weak absorption of
low intensity near about 285 nm and having emax
less than 100 is a forbidden transition..
29
- Chromophores
- But the term chromophore was originally used to
denote a functional group or some other
structural feature, the presence of which imparts
a colour to a compound. - A functional group which exhibits absorption of
electromagnetic radiations in the visible or
ultraviolet region is called a chromophore
(colour loving).
29
30
Chromophore Excitation lmax, nm Solvent
CC p?p 171 hexane
CO n?pp?p 290180 hexanehexane
NO n?pp?p 275200 ethanolethanol
31
AUXOCHROME
- An auxochrome is a group which itself does
not act as a chromophore but when attached to a
chromophore it shifts the absorption maximum
towards longer wavelength along with an increase
in the intensity of absorption.
NH2
Benzene
Aniline
?max 254nm max203 nm
? max 280 nm max1430 nm
32
Change in position and intensity of absorption
1) Bathochromic shift or red shift It involves
the shift of absorption maximum towards longer
wavelength. It can be brought about by three
different methods as given below a) By an
attachment of an auxochrome to the
chromophore, b) By conjugation of two or more
chromophoric groups, c) By using solvent of lower
polarity.
33
2)Hypsochromic shift or blue shift It involves
the shift of absorption maximum towards shorter
wavelength.It may be brought about as follows a)
By removal of conjugation in a system, c) By
using solvent of higher polarity.
3)Hyperchromic Effect This effect involves an
increase in the intensity of
absorption.It is brought about by introduction of
an auxochrome. 4)Hypochromic Effect This
effect involves a decrease in the intensity of
absorption. It is brought about by groups which
distort the geometry of the molecule.
34
HYPERCHROMIC
HYPSOCHROMIC
BATHOCHROMIC
HYPOCHROMIC
ABSORBANCE
? max
?
Terminology of shifts in the position of an
absorption band
35
Choice of solvents
- They need to be transparent and do not affect the
fine structure arising from the vibrational
effects
- Polar solvents generally tend to cause this
problem
- Same solvent must be used when comparing
absorption spectra for identification purpose.
36
Solvents for the Ultraviolet and Visible regions
Solvent Lower wavelength limit (nm) Solvent Lower wavelength limit (nm)
Water 180 Diethyl ether 210
Ethanol 220
Hexane 195
Cyclohexane 195
Carbon tetrachloride 260
37
Solvent effect
38
Effects of solvents
- 1. Blue shift (n- p) (Hypsochromic shift)
- Increasing polarity of solvent ? better solvation
of electron pairs (n level has lower E) - ? peak shifts to the blue (more energetic)
- 30 nm (hydrogen bond energy)
- 2.Red shift (n- p and p p) (Bathochromic
shift) - Increasing polarity of solvent, then increase the
attractive polarization forces between solvent
and absorber, thus decreases the energy of the
unexcited and excited states with the later
greater - ? peaks shift to the red
- 5 nm
39
Effect of polar solvent and shift in n - p
transition
p
p
?E1
n
?E2
Non- polar solvent
n
Polar solvent
?E1
?E2gt
40
Effect of polar solvent and shift in p - p
transition
p
?E1
p
?E2
p
Non- polar solvent
p
Polar solvent
?E1gt
?E2
41
Woodward-Fieser Rules
- This quantification is referred to as the
Woodward-Fieser Rules which we will apply to
three specific chromophores - Conjugated dienes
- Conjugated dienones
42
Woodward-Fieser Rules Dienes
The rules begin with a base value for ? max of
the chromophore being observed acyclic
butadiene 217 nm The incremental contribution
of substituents is added to this base value from
the group tables
Group Increment
Extended conjugation 30
Each exo-cyclic CC 5
Alkyl 5
-OCOCH3 0
-OR 6
-SR 30
-Cl, -Br 5
-NR2 60
43
Woodward-Fieser Rules - Dienes
- Isoprene - acyclic
butadiene 217 nm -
one alkyl subs. 5 nm
-
222 nm - Experimental
value 220 nm - Allylidene cyclohexane
- -
acyclic butadiene 217 nm - one
exocyclic CC 5 nm - 2 alkyl
subs. 10 nm -
232 nm -
Experimental value 237 nm
44
There are two major types of cyclic dienes, with
two different base values Heteroannular
(transoid) Homoannular (cisoid) e 5,000
15,000 e 12,000-28,000 base ?max 214
base ?max 253 The increment table
is the same as for acyclic butadienes with a
couple additions
Woodward-Fieser Rules Cyclic Dienes
Group Increment
Additional homoannular 39
Where both types of diene are present, the one with the longer l becomes the base
45
Woodward-Fieser Rules Cyclic Dienes
- In the pre-NMR era of organic spectral
determination, the power of the method for
discerning isomers is readily apparent - Consider abietic vs. levopimaric acid
abietic acid
levopimaric acid
46
- Woodward-Fieser Rules Cyclic Dienes
heteroannular diene 214 nm 4 alkyl subs. (4 x
5) 20 nm 1 exo CC 5 nm 239 nm
homoannular diene 253 nm 4 alkyl subs. (4 x
5) 20 nm 1 exo CC 5 nm
278 nm
47
- Woodward-Fieser Rules Cyclic Dienes
- Be careful with your assignments three common
errors
This compound has three exocyclic double bonds
the indicated bond is exocyclic to two rings
This is not a heteroannular diene you would use
the base value for an acyclic diene
Likewise, this is not a homoannular diene you
would use the base value for an acyclic diene
48
Woodward-Fieser Rules - Enones
Group Increment
6-membered ring or acyclic enone Base 215 nm
5-membered ring parent enone Base 202 nm
Acyclic dienone Base 245 nm
Double bond extending conjugation 30
Alkyl group or ring residue a, b, g and higher 10, 12, 18
-OH a, b, g and higher 35, 30, 18
-OR a, b, g, d 35, 30, 17, 31
-O(CO)R a, b, d 6
-Cl a, b 15, 12
-Br a, b 25, 30
-NR2 b 95
Exocyclic double bond 5
Homocyclic diene component 39
49
- Woodward-Fieser Rules Enones
- Aldehydes, esters and carboxylic acids have
different base values than ketones
Unsaturated system Base Value
Aldehyde 208
With a or b alkyl groups 220
With a,b or b,b alkyl groups 230
With a,b,b alkyl groups 242
Acid or ester
With a or b alkyl groups 208
With a,b or b,b alkyl groups 217
Group value exocyclic a,b double bond 5
Group value endocyclic a,b bond in 5 or 7 membered ring 5
50
- Woodward-Fieser Rules Enones
- cyclic enone 215
nm - 2 x b- alkyl
sub (2 x 12) 24 nm - 239 nm
- Experimental value
238 nm - cyclic enone 215 nm
- extended conj. 30 nm
- b-ring residue 12 nm d-ring
residue 18 nm exocyclic double bond 5 nm - 280 nm
- Experimental 280 nm
51
THANK YOU
?