Chapter 2 Properties of Materials - PowerPoint PPT Presentation
Title: Chapter 2 Properties of Materials
1
Chapter 2Properties of Materials
-
-
2
Properties of Materials
- Objectives
- Be able to describe the composition of matter.
- Be able to describe the components of an atom.
- Be able to describe how a type of element is
determined. - Be able to describe the four categories of
chemical bonds.
3
Properties of Materials
- Objectives
- Be able to describe the 4 states of matter.
- Be able to describe the components of an atom.
- Be able to describe nucleation of grains.
- Be able to describe the seven different crystal
systems. - Be able to describe properties of materials that
are dependent on their crystal structure.
4
Properties of Materials
- Objectives
- Be able to describe how materials are affected
and respond to forces. - Be able to describe material properties and
methods of testing. - Be able to discuss material defects and
impurities. - Be able to briefly discuss iron and steel
manufacturing. - Be able to briefly discuss nonferrous metal
manufacturing.
5
Properties of Materials
- Structure of Matter
- If the atomic structure, bonding structure,
crystal structure, and their imperfections in the
material are known, then the properties of the
material can be determined.
6
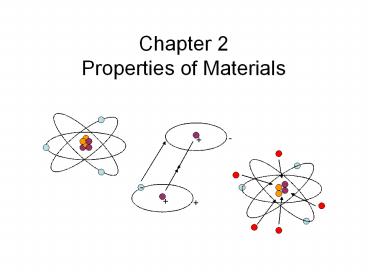
Properties of Materials
- Structure of Matter
- Matter is composed of atoms.
- Atoms are the smallest units of individual
elements. - Atoms are composed of protons, neutrons, and
electrons.
Electron (- charge)
Proton ( charge) Number of Protons determine
type of element
Neutron (No charge)
7
Properties of Materials
- Structure of Matter
- Atoms of the same element always have the same
number of protons, but the number of neutrons in
the nucleus may vary. - Atoms can combine to form molecules.
- Molecules are the smallest identifiable unit of
chemical compounds.
8
Properties of Materials
- Chemical Compound molecule atoms are held
together by chemical bonds. - Four types of bonds
- Ionic
- Covalent
- Metallic, and
- Van der Waal
9
Properties of Materials
-
- Ionic Bonds
- Created between charged particles
- Metallic elements have more electrons than their
most stable configuration. - Other, nonmetallic elements become more sable
with the addition of electrons to their atoms. - In this case, atoms having more electrons will
donate electrons to atoms needing electrons to
create a stable configuration. When this occurs
the atom with excessive electrons is called a
donating atom. - Donating atoms have more protons than electrons
and possess a positive charge. Positive charged
ion. - Where as, those atoms accepting electrons acquire
a negative charge. Negative charged ion.Sodium
Chloride - Resulting in charged particles attracted to each
other by ionic bonds. - Such bonds exist in Sodium Chloride, potassium
chloride.
-
10
Properties of Materials
- Covalent Bonds
- Bonds between elements that have too many
electrons to be given off or too many to be added
for ionic bonds to form. - Electrons are shared to form more stable
compounds. - Shared electrons form covalent bonds.
- All organic and most bonded plastics involve the
covalent bonds.
Shared electrons
11
Properties of Materials
-
-
-
-
- Metallic Bonds
- Always formed between metals
- All metals have an excess of electrons over their
most stable configuration. - Metallic theory Elemental metal atoms form
electron Clouds, to which the nuclei are
attracted, thus the metallic bond.
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
12
Properties of Materials
- Van der Waal Bonds
- Asymmetrical atoms or molecules with a net polar
moment in the charge. - Know as London Forces or Polar Bonds.
- Do not exist by themselves.
- Compounds like helium, two protons, two neutrons,
and two electrons which is a very stable gas
atom. But, at approximately -425 oF helium is
slowed and becomes asymmetrical forming a polar
bond. At this temperature helium forms a solid
due to the Van der Waal bond.
-
-
-
-
13
Properties of Materials
- Material Properties often depend on the type of
bonds in the material. - Melting Point
- Boiling Point
- Optical Transparency
- Electrical Conductivity
- Thermal Conductivity
- Crystal Structure
14
Properties of Materials
- All matter exists in one of four (4) states
- Gas,
- Liquid,
- Solid, and
- Plasma.
- In manufacturing it is important to know how
materials transition from liquids to solids
because these transitions are used in material
removal, forging, and heat treatments.
15
Properties of Materials
- Gaseous State
- A state of matter where the individual atoms or
molecules have little or no attraction to each
other. - Atoms or molecules are in constant motion.
- This movement is the result of the energy in the
material and a function of the heat stored within
the atoms. - These particles are continuously bouncing from
one particle to another or the gas container
walls. - These collisions result in heat generation
because of the energy in the material and is a
function of the heat stored in the atoms. - Compressing gases results in shoving particles
closer together, more collisions, and higher
temperatures and conversely. - May be comprised of individual atoms or
molecules. - Many gases are diatomic molecules where two atoms
bond to form an individual gas molecule, i.e.
oxygen (O2), hydrogen (H2), nitrogen (N2). - Very few items are manufactured from gases.
- Gases are used in process operations such as
welding.
16
Properties of Materials
- Boiling Point
- Gaseous State
- Temperature where the gas has been cooled to the
point that molecules or atoms begin to bond to
each other. - When a gas has been cooled to its boiling point,
then the heat of vaporization must be removed to
transform the as to a liquid. - Conversely, a liquid requires an addition of the
same energy (heat of vaporization) to transform
the liquid to a gas.
17
Properties of Materials
- Liquid State
- Liquids lack a definite long-range crystalline
structure. - Particles are arranged randomly.
- Produces bonds of various lengths.
- Longer the bond the weaker and more susceptible
to heat fracture reducing viscosity of the
material. - The more heat applied, shorter bonds will break.
- Rigid materials are not necessarily solids. Could
be a very viscous liquid. - Solids must possess a definite structure for
Material scientists.
18
Properties of Materials
- Solid State
- The material state which has a definite,
long-range crystalline structure. - Melting point of a solid is when a temperature is
reached where the bonds between particles can no
longer hold the particles together. - If enough energy is provided to break one bond,
then there is enough energy to break all the
bonds, and a melting point has been established. - All True solids have a definite melting point.
This is a test of a solid. - Glass is not a solid. It is a superviscous liquid
at room temperature.
19
Properties of Materials
- Nucleation of Grains
- Phenomenon when the temperature of a molten metal
is lowered to the melting point and small
crystals or nuclei are formed at many points in
the molten liquid. The nuclei form individual
crystals and start growing by adding more and
more atoms from the liquid to the solid.
Nucleus
Atoms or molecules
20
Properties of Materials
- Formation of grains in a solid
- Eventually, crystals or nuclei grow together
forming grains in a solid. - If the solid is maintained at temperatures just
below the melting temperature, then the large
grains will absorb the smaller grains in the
solid.
Solid nuclei
Melted Material
Melt
Nuclei formed
Nuclei growth
Solidification (Nuclei grow together)
21
Properties of Materials
- Crystal Structure
- Only seven different patterns or crystal systems
- Cubic
- Tetragonal
- Orthorhombic
- Rhombohedral
- Hexagonal
- Monoclinic
- Triclinic
- Fourteen modifications of these seven crystal
structures can occur.
22
Properties of Materials
- Additional Crystal Formations
- In addition to the fourteen previously identified
crystal modifications, there exists material
structures that overlap each other, i.e. diamonds
have Cubic close pack (complex cubic) and
graphite has Hexagonal close pack structures. - Some crystals grow large enough to be see by the
naked eye. But, most require the use of a
microscope to identify the crystal structure. - Regardless of the size the crystals will all have
the same shape. - Regardless of the size of the crystal, when
broken, they will always break into smaller
replicas of the large crystal.
23
Properties of Materials
- Properties of materials are dependent on their
crystal structure. - Examples of numerous material properties
- Density,
- Ductility,
- Malleability,
- Ultimate tensile strength,
- Yield strength in tension,
- Elongation,
- Modulus of elasticity,
- Compression strength,
- Shear\strength,
- Impact strength, and
- Fatigue strength.
24
Properties of Materials
- Strength Properties
- Materials vary greatly in the magnitude of forces
they can withstand. - Calculations for the forces and strengths of
materials are published in American Institute of
Steel Construction (AISC) Manual for Steel
Construction, Structural Steel Detailing, and
Design of Welded Structures. - Forces applied to materials
- Stress defined as the load per unit cross
section of area. - Strain defined as the elongation of a specimen
per unit of original length.
25
Strength Properties
- Stress
26
Strength Properties
- Strain
27
Stress-Strain Diagram
- Elastic Region
- Proportional Limit
- Tensile Strength
- Rupture Strength
- Plastic Region
- Draw on Board
28
Safety Factor
- Divide the yield strength by the design load of
the object to determine the safety factor.
29
Load Types
- Compression
- Shear
- Torsion
- Flexure
30
Density
- density mass / volume
- More dense means more atoms per cubic inch.
31
Specific Gravity
- SG Density of a Material / Density of Water
(62.4 lbs/cubic foot or 1 gram/milliliter)
32
Properties of Materials
- Surface Properties
- Hardness
- Plays an important part in manufacturing.
- Makes cutting tools dull very quickly.
- Tests
- Rockwell
- Brinnell
- Vickers
- Other Tests
- Tukon
- Shore Scleroscope
- Mohrs
33
Time-Dependent Properties
- Impact
- Creep
- Fatigue
34
Properties of Materials
- Testing of Materials
- Destructive Testing Parts damaged by testing
methods. - Nondestructive Testing- Parts not damaged by
testing methods.
35
Properties of Materials
- Defects and Impurities
- Defects are properties of material that alters
or upsets the orderly arrangement of particles in
the crystal. - Point Defects
- Vacancies
- Holes
- Interstitials
- Substitutes
- Line Defects
- Plane Defects
- Inclusions
36
Properties of Materials
- Iron and Steel
- Used in so many applications of manufacturing.
- Steel is produced from Iron ore.
- Reduction of the ore to produce iron and
- The conversion of the iron into steel.
- Steel is produced from iron because of the high
carbon content of iron. - Steel is generate for high-strength steel
applications. - Converters used in converting iron into steel.
- 1) Open Hearth Converter.
- 2) Bessemer Converter
- 3) Electric-arc Converter
- 4) Oxygen lance Converter
- Burns off Carbon in pig iron
37
STEELS
- AISI/SAE designation system.
- First two numbers designate alloys
- Second two numbers designate of Carbon.
- See Table 2-3
38
Properties of Materials
- Nonferrous Materials
- Aluminum- most abundant metal on the earth.
- Used mostly as an alloy.
- Huge electricity power requirement for
production. - Bayer Process
- Magnesium- noted for its light weight.
- Like aluminum requires huge electrical power
consumption for production. - Must be located near salt water.
- Copper- best commercially available electrical
conductor. - Bronze Brass
- Titanium- maintains its strength at high
temperatures. - More expensive and
- Difficult to machine