Making Salts - PowerPoint PPT Presentation
1 / 23
Title:
Making Salts
Description:
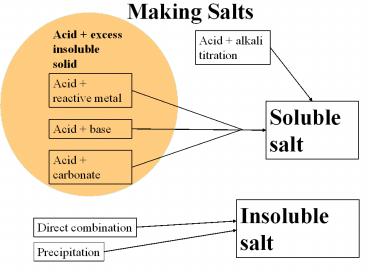
Making Salts Acid + excess insoluble solid Acid + alkali titration Acid + reactive metal Soluble salt Acid + base Acid + carbonate Insoluble salt Direct combination – PowerPoint PPT presentation
Number of Views:159
Avg rating:3.0/5.0
Title: Making Salts
1
Making Salts
Acid excess insoluble solid
Acid alkali titration
Acid reactive metal
Soluble salt
Acid base
Acid carbonate
Insoluble salt
Direct combination
Precipitation
2
Reactions of acids
Neutralisation of hydrochloric acid gives a
chloride sulphuric acid gives a sulphate
nitric acid gives a nitrate Acid metal ?
salt hydrogen Acid base ? salt water Acid
carbonate ? salt water carbon
dioxide Base A metal oxide or metal
hydroxide. An alkali is a water-soluble
base.
3
Salts
A salt is an ionic compound which is neither an
acid (containing H) or a base (containing O2- or
OH-). A salt is formed whenever an acid is
neutralised.
Acids, containing H
Salts
Ionic compounds
Bases, containing OH- or O2-
4
Uses of Salts
- Plant fertilisers (NPK)
- (NH4)2SO4, NH4NO3, KH2PO4, .
- Food flavour and preservation
- NaCl, KCl, KNO3.
- In toothpaste
- NaF
- Photographic film
- AgCl, AgBr
5
Salts are important also in
- Proteins and enzymes
- The molecules that carry out the chemical
reactions of life - DNA and RNA
- the molecules of inheritance
- Transmission of electrical impulses along nerves
6
Acid Neutralisation reactions
Neutralisation of hydrochloric acid gives a
chloride (containing Cl-) sulphuric acid gives a
sulphate (containing SO42-) nitric acid gives a
nitrate (containing NO3-) Acid metal ? salt
hydrogen Acid base ? salt water Acid
carbonate ? salt water carbon dioxide
7
Methods of salt making (1)
Acid alkali ? salt water HCl (aq)
NaOH (aq) ? NaCl (aq) H2O (l)
Titration of acid against alkali for soluble
salts of group 1 metals and ammonium 5 cm3
sodium hydroxide solution was measured using a
pipette and transferred to the conical flask. 2
drops of methyl orange indicator were added.
Hydrochloric acid was poured into the burette and
the starting reading was recorded. The acid was
added to the sodium hydroxide, drop by drop,
until the indicator turned from yellow to orange
(or red). Charcoal was added to the mixture to
absorb the indicator and was removed by
filtration. Water was evaporated from the
filtrate, leaving sodium chloride crystals.
8
Methods of salt making (2)
- Acid excess insoluble solid
- For all other soluble salts
- Solid is either a moderately reactive metal, a
base or a carbonate - Excess solid to react with all the acid
- Removed by filtration
9
Methods of salt making (3)
- Precipitation reactions
- To make insoluble salts
- 2 solutions each containing one of the ions in
the salt are mixed. - Salt is precipitated
- Salt is filtered, residue is washed on filter
paper and dried.
10
Methods of salt making (4)
- Direct combination of the elements
- sodium (l) chlorine (g) ? sodium chloride (s)
- 2 Na (l) Cl2 (g) ? 2 NaCl (s)
- aluminium (s) bromine (l) ? aluminium bromide
(s) - 2 Al (s) 3 Br2 (l) ? 2 Al Br3
(s)
11
Methods of salt making (1)
(a)
barium hydroxide (aq) nitric acid (aq)
Ba(OH)2 (aq) HNO3 (aq)
2
barium nitrate (aq) water (l)
Ba(NO3)2 (aq) H2O (l)
2
12
Methods of salt making (1)
(b)
ammonium carbonate (aq) sulphuric acid (aq)
(NH4)2CO3 (aq) H2SO4 (aq)
ammonium sulphate (aq) water (l) carbon
dioxide (g)
(NH4)2SO4 (aq) H2O (l) CO2 (g)
13
Methods of salt making (1)
(c)
ammonium hydroxide (aq) nitric acid (aq)
NH4OH (aq) HNO3 (aq)
ammonium nitrate (aq) water (l)
NH4NO3 (aq) H2O (l)
14
Methods of salt making (1)
(e)
potassium hydroxide (aq) nitric acid (aq)
KOH (aq) HNO3 (aq)
potassium nitrate (aq) water (l)
KNO3 (aq) H2O (l)
15
Methods of salt making (2)
Acid insoluble base ? salt water H2SO4
(aq) CuO (s) ? CuSO4 (aq) H2O (l)
16
Methods of salt making (2)
(g)
calcium (s) nitric acid
(aq)
Ca (s) HNO3 (aq)
2
calcium chloride(aq) hydrogen (g)
Ca(NO3)2 (aq) H2 (g)
17
Methods of salt making (2)
(f)
iron (s) sulphuric acid
(aq)
Fe (s) H2SO4 (aq)
iron (II) sulphate (aq) hydrogen (g)
FeSO4 (aq) H2 (g)
18
Methods of salt making (2)
(d)
calcium carbonate (s) nitric acid (aq)
2
CaCO3 (s) HNO3 (aq)
calcium nitrate (aq) water (l) carbon dioxide
(g)
Ca(NO3)2 (aq) H2O (l) CO2 (g)
19
Acid metal ? salt hydrogen
Magnesium hydrochloric acid ? magnesium
chloride hydrogen Mg (s) 2 HCl (aq)
? MgCl2 (aq) H2 (g)
Mg (s)
2 H 2 Cl- (aq)
?
Mg2 2 Cl- (aq)
H2 (g)
2 e-
copper hydrochloric acid ? no reaction Cu (s)
2 HCl (aq)
Cu (s)
2 H 2 Cl- (aq)
20
Metal Reactivity Series
Potassium Sodium Lithium Calcium Magnesium Zinc Ir
on Lead Hydrogen Copper Silver Gold Platinum
React with cold water
React with dilute acids and steam
Reactivity
Do not react with dilute acids and steam
21
Acid base ? salt water
Sulphuric acid copper oxide ?
copper sulphate water H2SO4 (aq)
CuO (s) ? CuSO4 (aq)
H2O (l)
2 H SO42- (aq)
Cu2 O2- (s)
?
Cu2 SO42- (aq)
H2O (l)
Sulphuric acid copper hydroxide ? copper
sulphate water H2SO4 (aq) Cu(OH)2
(s) ? CuSO4 (aq) 2 H2O (l)
2 H SO42- (aq)
Cu2 2 OH- (s)
?
Cu2 SO42- (aq)
2 H2O (l)
22
Carbonic acid
- H2CO3 (aq)
- 2 H CO32- (aq)
- Present in fizzy drinks
- H2CO3 (aq) ? CO2 (g) H2O (l)
23
Acid carbonate ? salt water carbon dioxide
Sulphuric acid nickel carbonate ? nickel
sulphate water carbon dioxide H2SO4 (aq)
NiCO3 (s) ? NiSO4 (aq) H2O (l) CO2
(g)
2 H SO42- (aq)
Ni2 CO32- (s)
?
Ni2 SO42- (aq)
H2CO3 (aq)
H2O (l) CO2 (g)