BR.21 schema - PowerPoint PPT Presentation
Title:
BR.21 schema
Description:
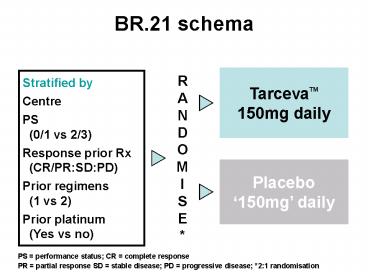
BR.21 schema TarcevaTM 150mg daily RANDOM I SE* Stratified by Centre PS (0/1 vs 2/3) Response prior Rx (CR/PR:SD:PD) Prior regimens (1 vs 2) Prior platinum – PowerPoint PPT presentation
Number of Views:79
Avg rating:3.0/5.0
Title: BR.21 schema
1
BR.21 schema
TarcevaTM150mg daily
RANDOM I SE
Stratified by Centre PS (0/1 vs 2/3) Response
prior Rx (CR/PRSDPD) Prior regimens (1
vs 2) Prior platinum (Yes vs no)
Placebo150mg daily
PS performance status CR complete response
PR partial response SD stable disease PD
progressive disease 21 randomisation
2
Study endpoints
- Primary
- overall survival
- Secondary
- progression-free survival (PFS)
- time to deterioration of cough, dyspnoea, painas
per EORTC QLQ-C30 QLQ-LC13 - response rates, duration
- toxicity and tolerability
- tissue HER1/EGFR versus outcome and safety
- TarcevaTM trough pharmacokinetics
HER/EGFR epidermal growth factor receptor
3
Key eligibility criteria
- Confirmed NSCLC, Stage IIIB or IV
- Age ³18 years
- PS 0, 1, 2 or 3
- Measurable or non-measurable disease
- One or two prior chemotherapy regimens
- Adequate organ function
- HER1/EGFR not required
- No prior HER1/EGFR inhibitors
- No prior malignancies or uncontrolled CNS M1
- Written informed consent
NSCLC non-small-cell lung cancer CNS M1
symptomatic central nervous system metastasis
4
BR.21 patient characteristics
Characteristic TarcevaTM(n488) Placebo(n243)
Median age (years) 62 59
Female () 35 34
PS 0, 1 () 13, 52 14, 54
PS 2, 3 () 26, 9 23, 9
Adenocarcinoma () 50 49
Prior regimens 1, 2, 3 () 50, 49, 1 50, 49, 1
Prior platinum () 93 92
Response to prior chemotherapy ()
CR/PR 40 40
SD 39 39
PD 21 21
Measurable disease () 88 87
5
Overall survival all patients
1.00 0.75 0.50 0.25 0
42.5 improvement in median survival
Survival distribution function
HR 0.73, plt0.001
HR 0.73, plt0.001
TarcevaTM Placebo
0 5 10 15 20 25 30
Survival time (months)
HR and p-value adjusted for stratification
factors at randomisation HER1/EGFR status
6
Survival after eliminating CR/PRs
- Exploratory analyses in BR.21 dataset
TarcevaTM TarcevaTM Placebo Placebo
n Median n Median HR p-value
SD/PD/NE 449 5.7 241 4.7 0.86 0.073
SD/PD 367 7.4 204 6.7 0.82 0.037
- The TarcevaTM benefit is still present after
eliminating CRs and PRs
7
Efficacy data for docetaxel, pemetrexed
erlotinib
Ramalingam, S. et al. Oncologist 200611655-665
8
BR.21 summary of significant clinical predictors
of response
ErlotinibPatients () (n427) p
Gender Female (146) 14.4 0.006
Gender Male (281) 6.1 0.006
Histology Adenocarcinoma (209) 13.9 lt0.001
Histology Other (218) 4.1 lt0.001
Ethnicity Asian (53) 18.9 0.02
Ethnicity Other (374) 7.5 0.02
Ever smoked Yes (311) 3.8 lt0.001
Ever smoked No (93) 24.7 lt0.001
Ever smoked Unknown (23) 13.0 lt0.001
Significance between subgroups
9
BR.21 Survival Across Subgroups
10
BR.21 Survival Across Subgroups Contd
11
Clinical Benefit of Erlotinib inMale Smokers
with SCCClark, Abstract 7166, Poster
NCIC CTG BR.21 Survival for Male, Ever Smokers
with SCC
Clark GM et al. ASCO 2006, Abs 7166.
12
BR.21 Symptom Benefit
Median time to deterioration (weeks) Median time to deterioration (weeks) Median time to deterioration (weeks) Median time to deterioration (weeks)
Parameter TarcevaTM Placebo Adjusted p-value
Cough 28.14 15.71 0.041
Dyspnoea 20.43 12.14 0.031
Pain 12.14 8.14 0.040
Adjustment for multiple testing
Patients were considered to have deteriorated
symptoms if the change in score from baseline for
each symptom was 10 points or higher at any
time-point after baseline assessment
13
QoL outcomes for second-line therapy
Agent Tool QoL outcomes Symptom outcomes
Tarceva1 EORTC QLQ-C30 QLQ-LC13 Significant improvement in global, physical, and emotional QoL versus BSC Significant increase in time to deterioration of symptoms (cough, dyspnoea, pain) versus BSC
Docetaxel2 Lung Cancer Symptom Scale (LCSS) No significant improvement for docetaxel 75mg/m2 compared with BSC
Pemetrexed3 LCSS No significant difference compared with docetaxel 75mg/m2
1 Bezjak A, et al. J Clin Oncol 20062438317 2
Dancey J, et al. Lung Cancer 20044318394 3
Hanna N, et al. J Clin Oncol 200422158997
14
BR.21 adverse events ()
TarcevaTM(n485) TarcevaTM(n485) Placebo(n242) Placebo(n242)
Any Grade 3, 4 Any Grade 3, 4
Rash 75 9 17 0
Diarrhoea 54 6 18 lt1
Nausea 33 3 24 2
Vomiting 23 2 19 2
Stomatitis 17 lt1 3 0
Fatigue 52 18 45 20
Ocular (all) 27 1 9 lt1
Anorexia 52 9 38 5
Infection 24 4 15 2
15
Comparison of phase III trials in relapsed
NSCLC haematological toxicity
Patients ()
Shepherd F, et al. N Engl J Med 200535312332
Hanna N, et al. J Clin Oncol 200422158997
16
ASCO 2006 Abstracts
17
1st-line Erlotinib in Elderly Patientswith
Advanced NSCLCJackman, Abstract 7168
Study Design Non-randomized, open label, Phase II
trial
Key inclusion criteria Endpoints Treatment
Age gt 70 years Stage IIIb/IV ECOG PS 0 2 gt3 wks since RT or major surgery Primary survival Secondary include RR, TTP, toxicity, QoL, symptom response Erlotinib 150 mg/day until PD or unacceptable toxicity Dose reductions (100 mg 50 mg) permitted for AEs
Jackman DM et al. ASCO 2006, Abs 7168.
18
1st-line Erlotinib in Elderly Patientswith
Advanced NSCLCJackman, Abstract 7168
Survival
Median Survival 41 weeks 52-week survival 40.4
Jackman DM et al. ASCO 2006, Abs 7168. Jackman
DM et al. 11th WCLC 2005, Abstract O-188.
19
1st-line Erlotinib in Elderly Patientswith
Advanced NSCLCJackman, Abstract 7168
Symptom Response
Poster
20
1st-line Erlotinib in Elderly Patientswith
Advanced NSCLCJackman, Abstract 7168
- Results
- For the 64 patients eligible for QoL analysis,
there was no statistically significant
improvement in overall LCSS score. - Patients who achieved PR or SD had statistically
significant improvements in their overall score
QoL as measured by LCSS. - Conclusions
- Patients over the age of 70 years had a median
survival of 10.9 months when treated with
erlotinib in the first-line. - Erlotinib in this population was also associated
with improvements in key symptoms of dyspnea,
cough, fatigue, pain and loss of appetite. - Improvements in overall LCSS score were noted in
patients who achieved disease control (PR or SD).
Jackman DM et al. ASCO 2006, Abs 7168.
21
1st-line Erlotinib in Advanced NSCLCwith Good
PrognosisAkerley, Abstract 7178
Study Design
Primary Objective Achieve 6-month
chemotherapy-progression-free survival rate that
is significantly higher than the historically
observed 31
Akerley W et al. ASCO 2006, Abs 7178.
22
1st-line Erlotinib in Advanced NSCLCwith Good
PrognosisAkerley, Abstract 7178
Response
Akerley W et al. ASCO 2006, Abs 7178.
23
1st-line Erlotinib in Advanced NSCLCwith Good
PrognosisAkerley, Abstract 7178
Overall Survival
Akerley W et al. ASCO 2006, Abs 7178.
24
1st-line Erlotinib in Elderly Patientswith
Advanced NSCLCAkerley, Abstract 7178
- Conclusions
- The overall response rate was 15 the 6-month
PFS rate is 56. - Rash predicts the duration of erlotinib
effectiveness. - Never smokers show a better survival outcome than
ever smokers. - Survival and PFS in this population of minimally
selected patients appear comparable to that with
chemotherapy. - A randomized trial is warranted to further
investigate the results of this trial.
Akerley W et al. ASCO 2006, Abs 7178.