Collision diameter - PowerPoint PPT Presentation
Title:
Collision diameter
Description:
Collision diameter The closest distance of approach between the centres of the molecules taking part in a collision is called collision diameter.it is usally ... – PowerPoint PPT presentation
Number of Views:117
Avg rating:3.0/5.0
Title: Collision diameter
1
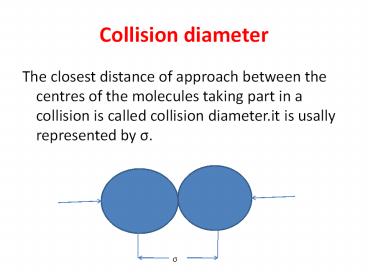
Collision diameter
- The closest distance of approach between the
centres of the molecules taking part in a
collision is called collision diameter.it is
usally represented by s.
s
2
Collision Number and Collision frequency
- The no of collision which a single molecules
makes with other molecules in one second is
called collision number. - On the basis of kinectic theory of gases,it can
be shown that in gas containing n identical
molecules per cm3,the no of collision which a
single molecules will undergo with other
molecules in one sec is given by - Nc v2p?s2n
- Where vaverage velocity of the gas molecules in
cm per sec - smolecular diameter in cm
- nno of molecules/cm3
3
Movement of molecules A assuming other molecules
to be stationary
Consider a particular molecule A moving in a
particular direction .if average speed of the
molecule is v cm/sec,I twill travel a distance of
v cm in one second.if only A is supposed to move
and all other molecules are supposed to be
stationary, A will collide in 1 sec with all the
molecules whose centres lie within the cylinder
of length v cm and radius equal to molecular
diameter s as shown in fiqure.
4
Types of molecular collisions
- (a) (b)
(c) - Relative velocity0 Relative velocity2v
Relative velocityv2?