NOMENCLATURE - PowerPoint PPT Presentation
1 / 2
Title:
NOMENCLATURE
Description:
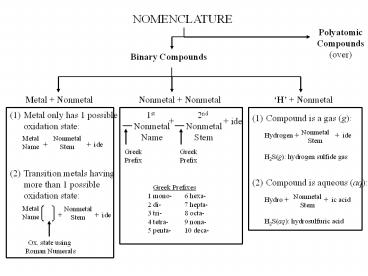
NOMENCLATURE Polyatomic Compounds (over) Binary Compounds Metal + Nonmetal Nonmetal + Nonmetal H + Nonmetal (1) Metal only has 1 possible oxidation state: – PowerPoint PPT presentation
Number of Views:113
Avg rating:3.0/5.0
Title: NOMENCLATURE
1
NOMENCLATURE
Polyatomic Compounds (over)
Binary Compounds
Metal Nonmetal
Nonmetal Nonmetal
H Nonmetal
(1) Metal only has 1 possible oxidation state
1st Nonmetal Name
2nd Nonmetal Stem
(1) Compound is a gas (g)
ide
Greek Prefix
Greek Prefix
H2S(g) hydrogen sulfide gas
- Transition metals having
- more than 1 possible
- oxidation state
- Compound is aqueous (aq)
Greek Prefixes 1 mono- 6 hexa- 2 di- 7
hepta- 3 tri- 8 octa- 4 tetra- 9 nona- 5
penta- 10 deca-
H2S(aq) hydrosulfuric acid
Ox. state using Roman Numerals
2
Polyatomic Compounds
H Poly. Anion Must learn parent acids then
Poly. Cation Simple Anion Poly. Cation Poly.
Anion
Metal Poly. Anion
Acid 1 more oxygen than parent per _____ic
acid Parent _____ic acid 1 less oxygen
than parent _____ous acid 2 less oxygens
than parent hypo_____ous acid When Hs are
replaced by metals, salts are derived
and per _____ic becomes per _____ate _____ic bec
omes _____ate _____ous becomes _____ite hypo
____ous becomes hypo ____ite
Only one poly. cation NH4 Simply name each
ion. NH4Cl ammonium chloride (NH4)2CO3
ammonium carbonate
Simple Anion
Poly. Anion
NaClO3 sodium chlorate FeSO4 iron(II) sulfate