Equilbrium Constant and EXTERNAL EFFECTS - PowerPoint PPT Presentation
1 / 17
Title:
Equilbrium Constant and EXTERNAL EFFECTS
Description:
Title: CHEMICAL EQUILIBRIUM Chapter 16 Author: J. Kotz Last modified by: Human01 Created Date: 6/20/1996 10:40:16 AM Document presentation format – PowerPoint PPT presentation
Number of Views:53
Avg rating:3.0/5.0
Title: Equilbrium Constant and EXTERNAL EFFECTS
1
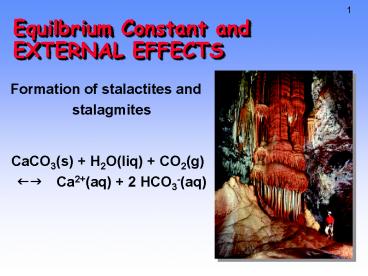
Equilbrium Constant and EXTERNAL EFFECTS
- Formation of stalactites and stalagmites
- CaCO3(s) H2O(liq) CO2(g)fg Ca2(aq) 2
HCO3-(aq)
2
EQUILIBRIUM
- Temperature, catalysts, and changes in
concentration/ pressure affect equilibria. - The outcome is governed by LE CHATELIERS
PRINCIPLE - ...if a system at equilibrium is disturbed, the
system tends to shift its equilibrium position to
counter the effect of the disturbance.
3
Equilibrium constant and Concentration
- Concentration changes
- no change in K
- only the position of equilibrium changes.
4
Butane-Isobutane Equilibrium
butane
isobutane
5
Butane Isobutane
butane
isobutane
- At equilibrium with iso 1.25 M and butane
0.50 M. K 2.5. - Add 1.50 M butane.
- When the system comes to equilibrium again, what
are iso and butane?
6
Butane e Isobutane
- Solution
- Calculate Q immediately after adding more butane
and compare with K.
Q is LESS THAN K. Therefore, the reaction will
shift to the ____________.
7
Butane e Isobutane
- Q is less than K, shifts right
- toward isobutane.
- Set up ICE table
- butane isobutane
- Initial
- Change
- Equilibrium
0.50 1.50
1.25
- X X
2.00 x 1.25 x
8
Butane e Isobutane
x 1.07 M At the new equilibrium position,
butane 0.93 M and isobutane 2.32 M.
Equilibrium has shifted toward isobutane.
9
Equilibrium Constant and Catalyst
- Add catalyst NO change in K
- A catalyst only affects the RATE it approach
equilibrium.
Catalytic exhaust system
10
Pressure and EquilibriumN2O4(g) e 2 NO2(g)
- Increase P in the system by reducing the volume
(at constant Temp).
e
11
- N2O4(g) e 2 NO2(g)
- Increase P in the system by reducing the volume.
- In gaseous system the equilibrium will shift to
the side with fewer molecules (in order to reduce
the P). - Therefore, reaction shifts LEFT and P of NO2
decreases and P of N2O4 increases.
12
Temperature Effects on Equilibrium
Figure 16.6
13
Temperature Effects on Equilibrium
- N2O4 (colorless) heat
- e 2 NO2 (brown) ?Ho 57.2
kJ (endo)
Kc (273 K) 0.00077 Kc (298 K) 0.0059
14
Every T has a unique K
- Temperature change change in K
- Consider the fizz in a soft drink
- CO2(aq) HEAT e CO2(g) H2O(l)
- K P (CO2) / CO2
- Increase T. What happens to equilibrium position?
To value of K? - K increases as T goes up because P(CO2) increases
and CO2 decreases. - Decrease T. Now what?
- Equilibrium shifts left and K decreases.
15
NH3 Production
- N2(g) 3 H2(g) e 2 NH3(g) heat
- K 3.5 x 108 at 298 K
16
Le Chateliers Principle
- Change T - changes K
- causes change in P or concentrations at
equilibrium - Use a catalyst K not changed.
- Reaction comes more quickly to equilibrium.
- Add or take away reactant or product K does not
change - Reaction adjusts to new equilibrium position
17
Examples of Chemical Equilibria
- Phase changes such as H2O(s)
H2O(liq)
e