2NH3 CO2 H2O ?(NH4) 2CO3 A - PowerPoint PPT Presentation
1 / 20
Title: 2NH3 CO2 H2O ?(NH4) 2CO3 A
1
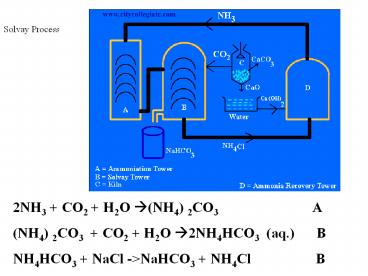
Solvay Process
2NH3 CO2 H2O ?(NH4) 2CO3
A (NH4) 2CO3 CO2 H2O
?2NH4HCO3 (aq.) B NH4HCO3 NaCl -gtNaHCO3
NH4Cl B
2
- NaHCO3 is used in
- water treatment
- as an additive in food and drinks eg baking
powder - for blowing foams such as expanded polystyrene
- in pharmaceutical products as an antacid
- in personal care products such as toothpaste and
- as an additive in animal feeds.
3
2NaHCO3 ? Na2CO3 CO2 H2 O Heavy soda ash 55
Glass containers 20 Chemicals, metallurgical,
detergents 25 Flat glass, glass fibre and other
glass Heavy soda ash Light soda ash 40 Heavy
chemicals (Phosphates, silicates and
chromates) 30 Food, drinks, detergents, textiles
and miscellaneous 30 Brine treatment and water
purification
4
The overall reaction 2NaCl(aq) CaCO 3 (s) Na 2
CO 3 (aq) CaCl 2 (aq) is endothermic (H 20
kJ mol 1 , G 60 kJ mol 1 ) and the
equilibrium lies well to the left. So the
production of sodium carbonate must be undertaken
by an indirect route. The actual series of
reactions used is 1) CaCO 3 (s) ?CaO(s) CO 2
(g) ?H 178 kJ mol 1 2) 2NaCl(aq) 2NH 3 (aq)
2H 2 O(l) 2CO 2 (g)? 2NH 4 Cl(aq) 2NaHCO 3
(s) ? H -158 kJ mol 1 3) 2NaHCO3(s)
?Na2CO3(s) CO2(g) H2O(l) ? H 85 kJ mol
1 4) CaO(s) H 2 O(l) ?Ca(OH)2 (s) ?H -65
kJ mol 1 5) Ca(OH)2 (s) 2NH 4Cl(aq) ?CaCl 2
(aq) 2NH 3 (aq) 2H 2 O(l) ?H
-20 kJ mol 1
5
Biological CaCO3
6
Calcitic microlenses as part of the photoreceptor
system in brittlestars
Joanna Aizenberg, Alexei Tkachenko, Steve
Weiner, Lia Addadi Nature 412, 819, 2001
7
Biomineralization on Organic Matrix
c
Aragonite
b
Acidic Macromolecule
a
Silk-fibroin-like proteins
ß-chitin
8
Asymmetry of biominerals in life
Chirality of Calcite Single Crystals in a
Coccoltih
9
The Concept of A Single Crystal in
Biomineralization
Pure synthetic calcite crystal
a intact spine from the sea urchin b fracture
surface of a young spine c fracture surface of a
mature spine
10
??????? Cleopatra
11
Pearl of Allah
40 Million
12
Germanium Ge
The element is a gray-white metalloid, and in its
pure state is crystalline and brittle.
GeO2 2C Ge 2CO GeO2 2H2 Ge
2H2O Very pure germanium can be made by the
reaction of GeCl4 with hydrogen. GeCl4 2H2
Ge 4HCl
Germanium was an element whose existence was
predicted by Mendeleev in 1871. He predicted that
the then unknown element germanium should
resemble silicon in its properties. He suggested
therefore the name ekasilicon (symbol Es).
Germanium was discovered in a mineral called
argyrodite by Clemens Alexander Winkler in 1886.
13
- It is stable in air under ambient conditions but
on heating in air or oygen, tin reacts with
oxygen to from tin dioxide, SnO2. - Sn(s) O2(g) SnO2(s)
- It is stable to water under ambient conditions
but on heating with steam, tin reacts with water
to from tin dioxide, SnO2 and hydrogen. - Sn(s) 2H2O(g) SnO2(s) 2H2(g)
- Chlorides
- SnCl2 tin (II) chloride Ionic
- SnCl4 tin (IV) chloride Covalent
- Alloy Tin is used to form many useful alloys.
Bronze is an alloy of tin and copper
14
Lead
General InformationLead is stable to air and
water, but will tarnish in moist air over long
periods. It dissolves in nitric acid. Lead is a
poor conductor of electricity.
15
- Lead is a soft, malleable and corrosion resistant
material. - Lead is used to line tanks that store corrosive
liquids, such as sulfuric acid (H2SO4). - Lead's high density makes it useful as a shield
against X-ray and gamma-ray radiation and is used
in X-ray machines and nuclear reactors. - Lead is also used as a covering on some wires and
cables to protect them from corrosion, as a
material to absorb vibrations and sounds and in
the manufacture of ammunition. - Most of the lead used today is used in the
production on lead-acid storage batteries, such
as the batteries found in automobiles.
16
Roman baths such as these in Bath, England, used
lead pipes for water
The ancient Romans used lead to make water pipes,
some of which are still in use today.
Unfortunately for the ancient Romans, lead is a
cumulative poison and the decline of the Roman
empire has been blamed, in part, on lead in the
water supply.
17
? ?!? ?! ???????!
18
?? ??????
19
Lead (II) oxide
20
In chemistry, the inert pair effect occurs when
electrons are pulled closer to the nucleus,
making them stabler and more difficult to ionise.
This is a relativistic effect. An electron
around the nucleus requires sufficient kinetic
energy in order not to be pulled towards the
nucleus. This results in it having higher speeds,
with a higher force acting on it by the nucleus.
The effects for the heavier elements are
appreciable, as electrons travel closer to the
speed of light, c. The s-orbital electrons are
more affected in this way since they have a
greater penetrating power. The mass of the
electron depends on its speed, given by its rest
mass multiplied by the Lorenz factor. Electrons
in heavier elements thus have greater increases
in their relativistic masses. The consequence of
this is that the Bohr radius is decrease. As the
electrons are pulled closer to the nucleus by
this effect, they are stabilised and harder to
ionise. This is called the inert pair effect.