Stoichiometry Objectives - PowerPoint PPT Presentation
1 / 67
Title:
Stoichiometry Objectives
Description:
Title: Stoichiometry Objectives Author: Administrator Last modified by: tprescott Created Date: 4/18/2006 4:09:10 PM Document presentation format – PowerPoint PPT presentation
Number of Views:382
Avg rating:3.0/5.0
Title: Stoichiometry Objectives
1
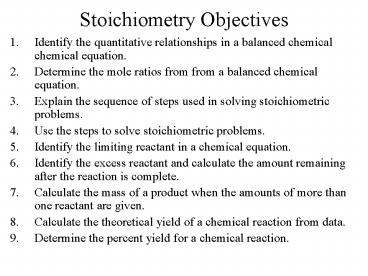
Stoichiometry Objectives
- Identify the quantitative relationships in a
balanced chemical chemical equation. - Determine the mole ratios from from a balanced
chemical equation. - Explain the sequence of steps used in solving
stoichiometric problems. - Use the steps to solve stoichiometric problems.
- Identify the limiting reactant in a chemical
equation. - Identify the excess reactant and calculate the
amount remaining after the reaction is complete. - Calculate the mass of a product when the amounts
of more than one reactant are given. - Calculate the theoretical yield of a chemical
reaction from data. - Determine the percent yield for a chemical
reaction.
2
The Mole (Ch 11)
- Chemists need a convenient method for counting
accurately the number of atoms, molecules, or
formula units in a sample of a substance. - -atoms and molecules are extremely small
- -even in the smallest sample its impossible
to actually count each individual atom. - To fix this problem chemists created their own
counting unit called the mole. - -mole commonly abbreviated mol, is the SI
base unit used to measure the amount of a
substance
3
The Mole
- Through experimentation, it has been established
1 mole 6.022 136 7 x 1023 representative
particles. - -representative particle any particle such
as atoms, molecules, formula units, electrons, or
ions. - -called Avogadros number in honor of the
Italian physicist and lawyer Amedeo Avogadro who,
in 1811, determined the volume of one mole of a
gas. - -we round Avogadros number to three
significant figures 6.02 x 1023. - - If you write out Avogadros number, it
looks like this - - 602 000 000 000 000 000 000 000
4
One Mole Quantities
5
Other Mole Stoichiometry Vocabulary
- 1. What is stoichiometry?
Study of quantitative relationships among amounts
of reactants and products.
- What is a mole ratio?
Ratio between the moles of any two substances in
a balanced chemical equation
3. What is molar mass?
Mass, in grams, of one mole of pure substance.
-numerically equal to its atomic mass -has
the units g/mol
6
Converting Moles to Particles (11.1)
- Determine how many particles of sucrose are in
3.50mol of sucrose. - - write a conversion factor using Avogadros
number that relates representative particles to
moles of a substance.
7
Converting Particles to Moles (11.1)
- Now, suppose you want to find out how many moles
are represented by a certain number of
representative particles, such as 4.50 x 1024
atoms of zinc. - -You can use the inverse of Avogadros number
as a conversion factor.
8
Mole Practice 1
- Identify and calculate the number of
representative particles in each of the following
quantities. - 1. 2.15 moles of gold
- 2. 0.151 mole of nitrogen oxide
- 3. 11.5 moles of potassium bromide
- Calculate the number of moles of the substance
that contains the following number of
representative particles. - 4. 8.92 x 1023 atoms of barium
- 5. 5.50 x 1023 molecules of carbon monoxide
- 6. 2.66 x 1023 formula units of potassium iodide
9
Practice-Homework
- p 311 1-3 p 312 4a-c
- Determine the number of atoms in 2.50 mol Zn
- Determine the number of formula units in 3.25 mol
AgNO3 - Determine the number of molecules in 11.5 mol H2O
- Determine the number of moles in
- a. 5.75 x 1024 atoms Al
- b. 3.75 x 1024 molecules CO2
- c. 3.58 x 1023 formula units ZnCl2
10
Moles to Mass (11.2)
- To convert between moles and mass, you need to
use the atomic mass found on the periodic table. - Calculate the mass of 0.625 moles of calcium.
- -According to the periodic table, the atomic
mass of calcium is 40.078 amu, so the molar mass
of calcium is 40.078 g/mol.
11
Mass to Moles (11.2)
- How many moles of copper are in a roll of copper
that has a mass of 848g?
12
Practice-Homework
- p 316 11ab-12ab
- Determine the mass in grams of
- a. 3.57 mol Al
- b. 42.6 mol Si
- Determine the number of moles of
- a. 25.5 g Ag
- b. 300.0 g S
13
Mass to Atoms (11.2)
- To find the number of atoms in a sample, you must
first determine the number of moles. - Calculate the number of atoms in 4.77 g lead.
- Determine moles
14
Mass to Atoms (cont.)
- 2. Determine atoms
You can also convert from number of particles to
mass by reversing the procedure above and
dividing the number of particles by Avogadros
number to determine the number of moles present.
15
Atoms to Mass
- Example problem 11-5, p 318
- A party balloon has 5.50x1022 atoms of helium.
What is the mass in grams of the helium? - 5.50x1022 atoms He x 1 mol He
0.0914 mol He - 6.02 x1023 atoms He
- 0.0914 mol He x 4.00 g He 0.366 g He
- 1 mol He
16
Mole Practice 2
- How many atoms are in the following samples?
- 1. 1.24 g cobalt
- 2. 0.575 g cesium
- How many grams are in the following samples?
- 3. 4.16 x1023 atoms of radium
- 4. 1.50 x 1020 atoms of cadmium
17
Practice-Homwork
- p 316 11ab-12ab, p 318 13ab-14ab
18
Moles of Compounds (11.3)
- A mole of a compound contains as many moles of
each element as are indicated by the subscripts
in the formula. - -For example, a mole of ammonia (NH3) consists
of one mole of nitrogen atoms and three moles of
hydrogen atoms. - -the molar mass of the compound is found by
adding the molar masses of all of the atoms in
the representative particle.
Molar mass of NH3 1(molar mass of N) 3(molar
mass of H)
Molar mass of NH3 1(14.007 g) 3(1.008 g)
17.031 g/mol
19
Practice
- p 322 25
- P 318 13ab-14ab
- p322
- 25. Determine the molar mass of each of the
following - NaOH, CaCl2, Sr(NO3)2
- p318
- How many atoms are in
- a. 55.2 g Li b. 0.230 g Pb
- What is the mass of
- a. 6.02x1023 atoms Bi
- b. 1.00x1024 atoms Mn
20
- -Mole relationships from a formula (p 321)
- Determine the number of moles of aluminum ions in
1.25 moles of aluminum oxide (Al2O3). - First we need the ratio of Al ions to Al2O3.
- 2 mol Al ions
- 1 mole Al2O3
- 1.25 mol Al2O3 x 2 mol Al ions 2.50 mol Al
ions 1 mole Al2O3
21
- -Mole relationships from a formula (p 321)
- P321 20-21
- Determine the number of moles of chloride ions in
2.50 mol ZnCl2. - Calculate the number of moles of each element in
1.25 mole glucose (C6H12O6).
22
- -Mole to Mass for compounds ( p 323)
- What is the mass of 2.50 moles of allyl sulfide,
(C3H5)2S? - Calculate the mass of allyl sulfide.
- 6(12.01 g/mol) 72.06 g/mol
- 10(1.01 g/mol) 10.10 g/mol
- 1(32.07 g/mol) 32.07 g/mol
- 114.23 g/mol
23
- -Mole to Mass for compounds ( p 323)
- What is the mass of 2.50 moles of allyl sulfide,
(C3H5)2S? - 2. Convert the moles to mass.
- 2.50mol (C3H5)2S x 114.23g (C3H5)2S
- 1 mol
(C3H5)2S - 286g (C3H5)2S
24
Mass of Compound to Moles
- Calculate the number of moles of water that are
in 1.000 kg of water? - 1. Before you can calculate moles, you must
determine the molar mass of water (H2O).
molar mass H2O 2(molar mass H) molar mass O
25
- 2. Now you can use the molar mass of water as a
conversion factor to determine moles of water. - -Notice 1.000 kg is converted to 1.000 x 103 g
26
Practice
- p 323 27-28, p 324 30ab
- 27. What is the mass of 3.25 moles H2SO4?
- What is the mass of 4.35x10-2 moles of ZnCl2?
- Determine the number of moles present in each of
the following - a. 22.6 g AgNO3
- b. 6.50 g ZnSO4
27
Mole Practice 3
- Calculate the molar mass of the following
- C2H5OH
- HCN
- What is the mass of the following
- 2.25 moles of KMnO4
- 1.56 moles of H2O
- Determine the number of moles in the following
- 35.0 g HCl
- 254 g PbCl4
- What is the mass in grams of one molecule of the
following - 7. H2SO4
28
Percent Composition, Molecular Empirical
Formulas
- Recall that every chemical compound has a
definite compositiona composition that is always
the same wherever that compound is found. - The composition of a compound is usually stated
as the percent by mass of each element in the
compound, using the following process.
29
Percent Composition
- Example Determine the percent composition of
calcium chloride (CaCl2). - 1. Determine mass of each ion in CaCl2.
- -1mol CaCl2 consists of 1mol Ca2 ions and
2mol Cl- ions. - 1mol Ca2 ions x 40.08g Ca2 ions
40.08g Ca2 ions - 1mol Ca2
ions - 2mol Cl- ions x 35.45g Cl- ions 70.90g
Cl- ions - 1mol Cl- ions
30
Percent Composition
- Example Determine the percent composition of
calcium chloride (CaCl2). - 2. Calculate molar mass of CaCl2.
- - 40.08g Ca2 ions 70.90g Cl- ions
110.98 g CaCl2 - 1 mole CaCl2
1 mole CaCl2 - 3. Determine percent by mass of each element.
31
Percent Composition
- Example Determine the percent composition of
calcium chloride (CaCl2). - 3. Determine percent by mass of each element.
- Ca 40.08 g Ca2 x 100 36.11 Ca2
- 110.98 g CaCl2
- Cl 70.90 g Cl- x 100 63.89 Cl-
- 110.98 g CaCl2
- 4. Make sure your percent compositions equal
100. - 36.11 Ca2 63.98 Cl- 100
32
Practice
- p 331 43, 45
- Calculate the percent composition of sodium
sulfate (Na2SO4). - 45. What is the percent composition of phosphoric
acid (H3PO4).
33
Empirical Formula
- You can use percent composition data to help
identify an unknown compound by determining its
empirical formula. - -empirical formula-simplest whole-number ratio
of atoms of elements in the compound. - In many cases, the empirical formula is the
actual - formula for the compound.
- ?the empirical formula of sodium
chloride is Na1Cl1, - or NaCl, which is the true formula
- sometimes, the empirical formula is not the
actual - formula of the compound.
- ?the empirical formula for N2O4 (the
actual) is NO2.
34
Empirical Formula
- Example The percent composition of an unknown
compound is found to be 38.43 Mn, 16.80 C, and
44.77 O. Determine the compounds empirical
formula. - - Because percent means parts per hundred
parts, assume that you have 100 g of the
compound. - 1. Calculate the number of moles of each element
in the 100 g of compound.
35
Empirical Formula
- Example The percent composition of an unknown
compound is found to be 38.43 Mn, 16.80 C, and
44.77 O. Determine the compounds empirical
formula. - 1. Calculate the number of moles of each element
in the 100 g of compound.
36
Empirical Formula
- Example The percent composition of an unknown
compound is found to be 38.43 Mn, 16.80 C, and
44.77 O. Determine the compounds empirical
formula. - 2. The results show the following relationship
- 3. Obtain the simplest whole-number ratio of
moles - -divide each number of moles by the smallest
number of moles. - 0.6995 mol Mn 1.339 mol C 2.798 mol O
- 0.6995 mol 0.6995 mol
0.6995 mol - 1 2
4
37
Empirical Formula
- Example The percent composition of an unknown
compound is found to be 38.43 Mn, 16.80 C, and
44.77 O. Determine the compounds empirical
formula. - 3. Obtain the simplest whole-number ratio of
moles - Mn
C O - 1 2
4 - 4. Determine the empirical formula.
- MnC2O4
38
Practice
- p 333 46-47
- A blue solid is found to contain 36.84 N and
63.16 O. What is the empirical formula? - Determine the empirical formula for a compound
that contains 35.98 Al and 64.02 S.
39
Molecular Formula
- For many compounds, the empirical formula is not
the true formula. - -Chemists have learned, though, that acetic
acid is a molecule with the formula C2H4O2, which
is the molecular formula for acetic acid. - -molecular formula tells the exact number of
atoms of each element in a molecule or formula
unit of a compound. - Notice the molecular formula for acetic acid
(C2H4O2) has exactly twice as many atoms of each
element as the empirical formula (CH2O). - -The molecular formula is always a
whole-number multiple of the empirical formula.
40
Molecular Formula
- Example Determine the molecular formula for
maleic acid, which has a molar mass of
116.1g/mol. - 1. empirical formula of the compound
- -composition of maleic acid is 41.39 C,
3.47 H, and - 55.14 O (change the to g)
41
Molecular Formula
- Example Determine the molecular formula for
maleic acid. - 1. empirical formula of the compound
- -the ratio of CHO is 111, making the
empirical formula - CHO
- 2. calculate the molar mass of CHO (empirical
formula). - -29.01g/mol
- 3. Determine the molecular formula for maleic
acid,
42
Molecular Formula
- Example Determine the molecular formula for
maleic acid. - 3. Determine the molecular formula for maleic
acid, - -shows the molar mass of maleic acid is 4x
that of CHO. - 4. Multiply CHO by 4 to get C4H4O4
43
Practice
- p 335 51, 53
- 51. A substance has a chemical composition of
65.45 C, 5.45 H and 29.09 O. The molar mass
of the molecular formula is 110.0 g/mol.
Determine the molecular formula. - 53. A compound contains 46.68 N and 53.32 O.
It has a molar mass of 60.01 g/mol. What is the
molecular formula?
44
Empirical Formula from Mass
- You can also calculate the empirical formula of a
compound from mass of individual elements. - Example Determine the empirical formula for
ilmenite, which contains 5.41g Fe, 4.64g Ti and
4.65g O. - 1. Multiply the mass by molar mass to get moles
- 5.41g Fe x 1 mol Fe 0.0969 mol Fe
- 55.85 g Fe
- 4.64g Ti x 1 mol Ti 0.0969 mol Ti
- 47.88g Ti
- 4.65g O x 1 mol O 0.291 mol O
- 16.00g O
45
Empirical Formula from Mass
- Example Determine the empirical formula for
ilmenite, which contains 5.41g Fe, 4.64g Ti and
4.65g O. - 2. Multiply by the smallest number to get the
mole ratio. - 0.0969 mol Fe 0.0969 mol Ti 0.291 mol
O - 0.0969 mol 0.0969 mol
0.0969 mol - 1 1
3 - 3. Calculate the empirical formula.
- FeTiO3
46
Practice
- p 337 54-55
47
- TEST!!!!
48
Stoichiometry Practice Conservation of Mass
- For the following balanced chemical equations,
determine all possible mole ratios.
- HCl(aq) KOH(aq) ? KCl(aq) H2O(l)
- 2. 2Mg(s) O2(g) ? 2MgO(s)
49
Stoichiometric Calculations
- Many times we need to determine a certain amount
of product from a reaction or want to know how
much product will form from a given amount of
reactant.
To do this you need 1. balanced chemical
equations 2. mole ratios 3. molar mass
50
Stoichiometric Calculations mole-mole
- Example If you put 0.0400 mol of K into water,
how much hydrogen gas will be produced? - 2K(s) 2H2O(l) ? 2KOH(aq) H2(g)
- Use modified RICE table
- R Reaction (must be balanced)
- I Initial (amount in moles)
- C Change (also in moles)
- E End (really means equilibrium, but.)
51
Stoichiometric Calculations mole-mole
- Example If you put 0.0400 mol of K into water,
how many moles of hydrogen gas will be produced? - R 2K(s) 2H2O(l) ? 2KOH(aq)
H2(g) - (0.400 mol)
(_____ mol) - I 0.400 0
- C -0.400 1/2(0.400)
- E 0 0.200
- subtract from reactants, add to products
- products start at 0 mol
- what about water? it is in excess.
- why multiply by ½? b/c KH2 21 mole ratio
(1/2 as much H2 as K)
52
Stoichiometric Calculations Practice
- How many moles of carbon dioxide are produced
when 10.0 moles of propane (C3H8) are burned in
excess oxygen in a gas grill. Water is also a
product. - 2. Sulfuric acid is formed when sulfur dioxide
reacts with oxygen and water. Write the balanced
chemical equation for the reaction. If 12.5 mol
SO2 reacts, how many moles H2SO4 can be produced?
How many mole O2 is needed?
53
Practice
- p 359 10
- 10. CH4(g) S8(s) ? CS2(l) H2S(g)
- a. Balance the equation.
- b. Calculate moles of CS2 produced when
1.50 mol S8 is - used.
- c. How many mol H2S is produced?
54
Stoichiometric Calculations mole-mass
- Example If you put 0.0400 mol of K into water,
how many grams of hydrogen gas will be produced? - R 2K(s) 2H2O(l) ? 2KOH(aq)
H2(g) - (0.0400 mol)
(_____ g) - I 0.0400 0
- C -0.0400 1/2(0.0400)
- E 0 0.0200
- 0.0404 g
- must change moles into grams if asked for mass!!
55
Practice
- p 360 11-12
- If you begin with 1.25 mol TiO2, what mass of Cl2
is - needed? TiO2 C Cl2 ? TiCl4
CO2 - 12. Sodium chloride is decomposed into the
elements sodium and chlorine by means of
electrical energy. How many grams of chlorine
gas are produced from 2.50 mol sodium chloride?
56
Stoichiometric Calculations mass-mass
- Example If you put 15.0g of K into water, how
many grams of hydrogen gas will be produced? - R 2K(s) 2H2O(l) ? 2KOH(aq)
H2(g) - 15.0g
(_____ g) - I 0.384 0
- C -0.384
1/2(0.384) - E 0 0.192
- 0.388 g
- now we must change initial mass to moles!!
57
Practice
- p 361 13-14
- Determine the mass of N2 produced if 100.0g NaN3
is - decomposed. NaN3(s) ? Na(s) N2(g)
- 14. If 2.50 g sulfur dioxide reacts with excess
oxygen and water, how many grams of sulfuric acid
are produced?
58
Extra Practice
- p 379-380 61, 64, 70
59
- QUIZ!!!!
60
Limiting Reactants
- Rarely are the reactants in a chemical reaction
present in the exact mole ratios specified in the
balanced equation. - -usually, one or more of the reactants are
present in excess, and the reaction proceeds
until all of one reactant is used up. - -the reactant that is used up is called the
limiting reactant - The limiting reactant limits the reaction and,
thus, determines how much of the product forms. - - The left-over reactants are called excess
reactants
61
Limiting Reactants
- In the reaction below, 40.0 g of sodium hydroxide
(NaOH) reacts with 60.0 g of sulfuric acid
(H2SO4). - a. Calculate the limiting reactant
- b. Determine the reactant in excess
- c. Calculate the mass of water produced
- d. Calculate the mass of reactant in excess.
62
Limiting Reactants
- R
- 40.0g 60.0 g
(_____ g) - I 1.00 0.612 0
- C -1.00 -1/2(1.00)
1.00 - E 0 0.112
1.00 - change both initial masses to moles
- compare ratio, I need a NaOH H2SO4 of 2 1,
so ask - Do I have 2x as much NaOH as H2SO4?
- No, b/c 2(0.612) 1.22 and I only have
1.00, therefore - NaOH is my limiting reactant and H2SO4 is
excess.
63
Limiting Reactants
- R
- 40.0g 60.0 g
(_____ g) - I 1.00 0.612 0
- C -1.00 -1/2(1.00)
1.00 - E 0 0.112
1.00 - change mol product to mass
- show work
- to determine amount in excess, take moles excess
and change to mass - show work
64
Practice
- p 368 20-21
65
Percent Yield
- Most reactions never succeed in producing the
predicted amount of product. - -not every reaction goes cleanly or
completely - ?liquids may stick to surfaces of
containers - ?liquids may vaporize/evaporate
- ?solids may be left behind on filter paper
- ?solids may be lost in the purification
process - ?sometimes unintended products form
- The amount you have been calculating so far has
been the theoretical yield, the maximum amount of
product that can be produced from a given amount
of reactant.
66
Percent Yield
- A chemical reaction rarely produces the
theoretical yield. - -actual yield is the amount of product
produced in the chemical reaction - We can measure the efficiency of the reaction by
calculating the percent yield. - -percent yield ( yield) of the product is
the ratio of actual yield to the theoretical
yield, expressed as a percent. - yield actual yield (from the
experiment) x 100 - theoretical yield (from
calculations)
67
Percent Yield
- When potassium chromate is added to a solution
containing 0.500 g of silver nitrate, solid
silver chromate is formed. - a. Determine the theoretical yield of silver
chromate - b. If 0.455 g of silver chromate is actually
obtained, - calculate the percent yield.