Electromagnetic Radiation - PowerPoint PPT Presentation
Title:
Electromagnetic Radiation
Description:
For electrons in atoms, wave properties are important. deBroglie Equation: Matter waves Macroscopic object: 200 g rock travelling at 20 m/s has a wavelength: ... – PowerPoint PPT presentation
Number of Views:300
Avg rating:3.0/5.0
Title: Electromagnetic Radiation
1
(No Transcript)
2
Chapter 6 Electromagnetic Radiation
3
Figure 7.1
4
(No Transcript)
5
(No Transcript)
6
- Rank the following in order of increasing
frequency - microwaves
- radiowaves
- X-rays
- blue light
- red light
- UV light
- IR light
7
- Waves have a frequency
- Use the Greek letter nu, ?, for frequency, and
units are cycles per sec - All radiation ? ? c
- c velocity of light 3.00 x 108 m/sec
- Long wavelength --gt small frequency
- Short wavelength --gt high frequency
8
- What is the wavelength of WONY?
- What is the wavelength of cell phone radiation?
Frequency 850 MHz - What is the wavelength of a microwave oven?
Frequency 2.45 GHz
9
Quantization of Energy
Light acts as if it consists of particles called
PHOTONS, with discrete energy.
- Energy of radiation is proportional to frequency
E h ?
h Plancks constant 6.6262 x 10-34 Js
10
E h ?
Relationships
11
- Rank the following in order of increasing photon
energy - microwaves
- radiowaves
- X-rays
- blue light
- red light
- UV light
- IR light
12
E h n
What is the energy of a WONY photon?
13
Energy of Radiation
- What is the frequency of UV light with a
wavelength of 230 nm? - What is the energy of 1 photon of UV light with
wavelength 230 nm?
14
- What is the energy of a mole of 230 nm photons?
- Can this light break C-C bonds with an energy of
346 kJ/mol?
15
- Does 1200 nm light have enough energy to break
C-C bonds?
16
Where does light come from?
- Excited solids emit a continuous spectrum of
light - Excited gas-phase atoms emit only specific
wavelengths of light (lines)
17
Light emitted by solids
18
Light emitted by hydrogen gas
19
The Bohr Model of Hydrogen Atom
- Light absorbed or emitted is from electrons
moving between energy levels - Only certain energies are observed
- Therefore, only certain energy levels exist
- This is the Quanitization of energy levels
20
Emission spectra of gaseous atoms
- Excited atoms emit light of only certain
wavelengths - The wavelengths of emitted light depend on the
element.
21
Line spectra of atoms
22
Energy Adsorption/Emission
23
- For H, the energy levels correspond to
Constant 2.18 x 10-18 J
Energy level diagram
24
Each line corresponds to a transition
- Example n3 ? n 2
25
Explanation of line spectra
Balmer series
26
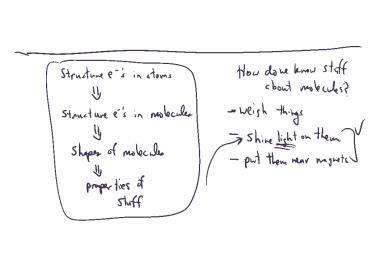
Matter Waves
- All matter acts as particles and as waves.
- Macroscopic objects have tiny waves- not
observed. - For electrons in atoms, wave properties are
important. - deBroglie Equation
27
Matter waves
Macroscopic object 200 g rock travelling at 20
m/s has a wavelength
Electron inside an atom, moving at 40 of the
speed of light
28
Can see matter waves in experiments
29
Heisenberg Uncertainty Principle
- Cant know both the exact location and energy of
a particle - So, for electrons, we DO know the energy well, so
we dont know the location well
30
Schrodingers Model of H
- Electrons act as standing waves
- Certain wave functions are allowed
- Wave behavior is described by wave functions ?
- ?2 describes the probability of finding the
electron in a certain spot - Also described as electron density
31
Example Wavefunction
- Equation slightly simplified
32
Its all about orbitals
- Each wavefunction describes a shape the electron
can take, called an ORBITAL - Allowed orbitals are organized by shells and
subshells - Shells define size and energy (n 1, 2, 3, )
- Subshells define shape (s, p, d, f, )
- Number of orbitals is different for each
subshell - s 1 orbital
- p 3 orbitals
- d 5 orbitals
- f 7 orbitals
33
(No Transcript)
34
Shells, Subshells and Orbitals
35
Which subshell does not exist?
- 5s
- 2p
- 2d
- 4f
- 15s
36
(No Transcript)
37
(No Transcript)
38
NODES
Spherical Nodes
39
Quantum Numbers and Numbers of Orbitals