Intravenous GP IIb/IIIa Inhibitors - PowerPoint PPT Presentation
Title:
Intravenous GP IIb/IIIa Inhibitors
Description:
Intravenous GP IIb/IIIa Inhibitors Abciximab (ReoPro) Has a rapid, high affinity to platelets within minutes Dissociation slowly from GP IIb/IIIa receptor Clears ... – PowerPoint PPT presentation
Number of Views:243
Avg rating:3.0/5.0
Title: Intravenous GP IIb/IIIa Inhibitors
1
(No Transcript)
2
Intravenous GP IIb/IIIa Inhibitors
- Abciximab (c7E3 Fab, ReoPro) Human- murine
chimeric monoclonal Fab antibody fragment - Eptifibatide (Integrilin) A cyclic
heptapeptide based on the Lys-Gly-Asp (KGD)
amino acid sequence - Tirofiban (Aggrastat) Tyrosine derivative
non-peptide mimetic inhibitor
3
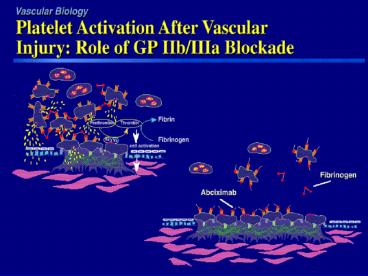
Intravenous GP IIb/IIIa InhibitorsAbciximab
(ReoPro)
- Has a rapid, high affinity to platelets within
minutes - Dissociation slowly from GP IIb/IIIa receptor
- Clears rapidly from plasma but remains bound to
circulating platelets up to 21 days - Binding to IIb/IIIa receptor is non-specific and
has equal affinity for the vitronectin receptor
which appears to play a role in cell adhesion,
migration and proliferation
4
Intravenous GP IIb/IIIa Inhibitors
Reopro Integrilin Aggrastat
Structure Monoclonal antibody Peptide Non-peptide Tyrosine derivative
Specificity to IIb/IIIa receptor Also binds to vitronectin Very Very
Platelet bound t1/2 Hours Seconds Seconds
Platelet recovery 12-36 Hrs 4 Hrs 4 Hrs
Plasma t1/2 Minutes 2.5 Hrs 1.8 Hrs
of dose in bolus 75 lt2-16 lt2-5
5
IV IIb/IIIa Inhibitors and Time course of Action
on Platelets
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
N 2099
N 1265
N 2792
11
(No Transcript)
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
EPISTENT
20
EPISTENT
Stent Placement
Placebo/Stent
Abx
/Stent
Abx
/Balloon
Placebo/Stent
Abx
/Stent
Abx
/Balloon
No. Patients
No. Patients
809
794
796
Stent Placed ()
95.3
96.9
Stent Placed ()
19.1
No. Stents/Pt ()
No. Stents/Pt ()
1
69.6
73.8
1
66.4
2
21.9
20.2
2
23.0
3
5.7
4.5
3
7.2
³
³
2.7
1.6
4
3.3
4
Maximum Balloon
Maximum Balloon
Inflation Pressure (mm)
16.0
16.0
Inflation Pressure (mm)
16.0
21
EPISTENT
o
Death, MI, or Urgent Intervention
p 0.051
p 0.007
p lt 0.001
p lt 0.001
Reduction
Reduction
vs
vs
Stent Only
Stent Only
Abciximab Stent
47
51
Abciximab Stent
47
51
Abciximab Only
24
37
Abciximab Only
24
37
22
EPISTENT
23
EPISTENT
24
EPISTENT
25
EPISTENT
26
EPISTENT
27
EPISTENT
28
EPISTENT
29
EPISTENT
30
World Wide Effect of Reopro if Used in All PTCA
Procedures - Extrapolation from EPISTENT
No. of Lives Saves gt2500 No. of MIs
Prevented gt40,000 No. of emergency
Revascularisation gt6500 Prevented
31
(No Transcript)
32
RESTORE Trial - Tirofiban for Patients with
UA or acute MI undergoing PTCA
12
Placebo
24
Tirofibin
10.5
p0.052
10
N 2139
)
8
8
Treatment was given for 36 hrs
)
Baseline characteristics or risk
)
stratification were not predictive
6
of treatment effect
Composite Endpoint (Death, MI, Urgent R
The differences in death and MI
)
rates was maintained over 6
months but the rate of
4
revascularisation remained
unchanged
Subgroup angiography study of
)
2
600 pts should no effect on
restenosis
0
Treatment Arms
R Revascularisation.
33
Overview of Randomised Trials of GP IIb/IIIa
Inhibitors During Coronary Intervention
34
RAPPORT TRIAL - Reopro For Primary
PTCA
35
N 483
p 0.038
0.02
48
30
28.2
28.1
25
20
Placebo
16.6
Event Rate ()
Reopro
15
11.2
9.5
10
5.8
5
0
Endpoint A
Endpoint B
Bleeding Complications
Endpoint A Death, recurrent MI, urgent repeat
TVR at 30 days. B Death, recurrent MI, any
repeat TVR (inc elective ones) at 6 month
35
GP IIb/IIIa Inhibitors for Coronary Intervention
- In gt15,000 patients GP IIb/IIIIa blockade shown
to reduce risk of important acute ischaemic
events by gt50-60 during coronary intervention - The treatment effect extends to each of the
components of the composite clinical endpoints
(Death, MI, and emergency revascularisation) - The inhibition of ischaemic events is achieved
early (12-48 hrs) and maintained up to 3 yrs
36
GP IIb/IIIa Inhibitors for Coronary Intervention
- All patients regardless of their demographics,
clinical, angiograhic, or procedural
characteristic benefit - Patients with UAP and Diabetics obtain the
greatest benefit - There may be heterogeneity among the different
IIb/IIIa inhibitors. The greatest impact has been
shown with Reopro. This may be due to its
non-specific binding ability and its different
pharmocokinetic profile
37
GP IIb/IIIa Inhibitors for Coronary Intervention
- Reopro may be specially effective in reducing TVR
and therefore restenosis in diabetes in
association with coronary stenting but influence
of GP IIb/IIIa inhibitors on restenosis in other
groups remain unclear - Safety of these drugs is increased by keeping ACT
200 during procedure and avoiding
post-procedural heparin with early sheath removal - Economical aspects of this therapy needs to be
evaluated
38
Who should have Reopro during Coronary
Intervention
- Patients with UAP at rest with ECG changes, or
Refractory angina or Post MI angina particularly
if - Diabetic
- Clot present
- TIMI 3 flow not achieved
- Troponin level is raised
- ? In Rescue / Salvage PTCA
39
EPISTENT
40
EPISTENT
41
EPISTENT
42
EPISTENT
43
EPISTENT
44
EPISTENT