Fixed Bed Reactor
1 / 65
Title: Fixed Bed Reactor
1
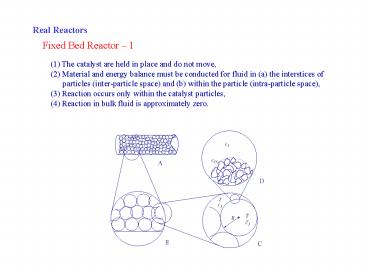
Real Reactors
Fixed Bed Reactor 1
(1) The catalyst are held in place and do not
move, (2) Material and energy balance must be
conducted for fluid in (a) the interstices of
particles (inter-particle space) and (b)
within the particle (intra-particle space), (3)
Reaction occurs only within the catalyst
particles, (4) Reaction in bulk fluid is
approximately zero.
2
Real Reactors
Fixed Bed Reactor 2
(5) Catalytic Reaction Steps (a) transport
of reactants and energy from bulk liquid to the
catalyst pellet surface, (b)
transport of reactants and energy from pellet
surface to pellet interior, (c) adsorption
of reactants, chemical reaction and desorption of
products at catalytic sites,
(d) transport of products from the pellet
interior to the surface, (e) transport of
products into the bulk fluid. - usually
one or at most two of the five steps are rate
limiting and dictate, - most often it is
the intra-particle transport step
3
Fixed Bed Reactors
Catalyst Bed
- Single pellet model is established by averaging
the microscopic processes that occur within the
intra-particle environment, - An effective diffusion coefficient is used to
- represent the information about the
- physical diffusion process
- and pore structure,
- A viable commercial catalyst must have sufficient
- active sites to maintain a product formation
rate - in the order of 1 mol/L h,
- Catalyst pellets usually takes the shape of
spheres - (0.3-0.7 cm), cylinders (0.3-1.3 cm O.D.
and - L/O.D. 3-4) and rings (ca. 2.5 cm)
4
Fixed Bed Reactors
General Balances Catalyst Particle
- Material Balance
- where
5
Fixed Bed Reactors
General Balances Catalyst Particle
- Energy Balance
- where
6
Fixed Bed Reactors
Catalyst
- Catalyst (usually metal sometimes also metal
oxides) is often dispersed onto large surface
area support material, - The support is often a refractor, metal oxide
such as alumina. Silica, clay, zeolite,
carbonaceous (e.g., activated carbon and
graphite) are also popular support material. - The support often have surface areas between
0.05-100 m2/g.
7
Fixed Bed Reactors
Catalyst Pellets 1
- Catalyst pellets are made by tableting and
extrusion methods. The latter is the more popular
method, - Different pellet shape and size could be obtained
by simply changing the extruder head, - The pellet shape and size could be optimized to
increase mass transfer rates, while minimizing
the pressure drop in the reactor.
8
Fixed Bed Reactors
Catalyst Pellets 2
- The pellet void fraction or porosity,
where rp is the effective pellet density
and Vg is the pore volume, - The pore volume range fro, 0.1-1 cm3/g pellet,
- The pellet can possess either a uniform pore size
or a bimodal pores of two different sizes, a
large size to facilitate transport and a small
size to contain the active catalyst sites.
9
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 1
- Material balance
- Steady-state
- Spherical coordinate system
10
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 2
- Boundary conditions
absence of driving force
11
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 3
- Dimensionless equation - 1
- characteristic length
- dimensionless length
dimensionless concentration
concentration scale
length scale
12
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 4
- Dimensionless equation 2
- where
13
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 5
- Simplification
- where
14
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 6
- General solution
- Specific solution
15
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 7
- Concentration profile in pellet
16
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 8
- Total productivity in pellet
- letting
17
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 9
- Effectiveness factor 1
- where
- 1 the entire pellet volume is reacting at the
same high rate because reactant is able to
diffuse quickly through the pellet, - 0 the pellet reacts at a slow rate, since the
reactant is unable to penetrate into the pellet
interior.
18
Single Pellet Reaction
First-Order Reaction (1) Spherical Pellet 9
- Effectiveness factor 2
19
Single Pellet Reaction
Example 1
The first order, irreversible reaction took place
in a 0.3 cm radius spherical catalyst pellet at T
450 K. At 0.7 atm partial pressure of A, the
pellets production rate is 2.5 x 10-5 mol/g-s,
what is the production rate at the same
temperature for a 0.15 cm radius catalyst pellet.
Given
20
Single Pellet Reaction
Example 2
- List the equations for (a) overall productivity,
(b) effectiveness factor and (c) Thiele modulus
for a first order reaction in a spherical pellet.
21
Single Pellet Reaction
Example 2
- Solve for Thiele modulus
- where
2.125 mol/cm3s (0.3 cm)2
0.007 cm2/s (1.9 x 10-5 mol/cm3)
k (0.3 cm)2
( )0.5
0.007 cm2/s
22
Single Pellet Reaction
Example 2
- Solve for overall productivity of a smaller
pellet
2.61/s (0.3 cm)2
( )0.5
0.007 cm2/s
The smaller pellet has about 60 better overall
productivity! Note this is only true when the
system is within diffusion-limited regime!
23
Single Pellet Reaction
First-Order Reaction Other Pellet Geometries 1
- Governing equation
24
Single Pellet Reaction
First-Order Reaction Other Pellet Geometries 2
- Characteristic Lengths
- Dimensionless equations
25
Single Pellet Reaction
First-Order Reaction Other Pellet Geometries 3
- Effectiveness factor 1
- or
26
Single Pellet Reaction
First-Order Reaction Other Pellet Geometries 4
- Effectiveness factor 2
27
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 5
- Positive reaction orders
- Redefining Thiele Modulus
28
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 6
- Redefining the equations
29
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 7
- Effectiveness factor as a function of Thiele
modulus
n ? 1
30
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 8
- Effectiveness factor as a function of Thiele
modulus
n lt 1
31
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 9
- Concentration profile within pellet with reaction
order less than 1
n 0
32
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 10
- Effectiveness factor can be approximated by the
analytical solution for first order reaction
n gt 0
concentration profile
effectiveness factor
overall productivity
33
Single Pellet Reaction
Other Reaction Orders Spherical Pellet 10
- Effectiveness factor can be approximated by the
analytical solution for first order reaction
n gt 0
concentration profile
effectiveness factor
overall productivity
34
Single Pellet Reaction
Hougen-Watson - 1
Find the effectiveness factor for a slab catalyst
geometry (1) Governing equation
35
Single Pellet Reaction
Hougen-Watson - 2
(2) Transformation into dimensionless
equation where
(dimensionless adsorption constant)
36
Single Pellet Reaction
Hougen-Watson - 3
(3) Effectiveness factor (4) Rescaling
the Theile modulus
37
Single Pellet Reaction
Hougen-Watson - 4
(5) Effectiveness factor versus Thiele modulus
38
Single Pellet Reaction
External Mass Transfer - 1
Rapid EMT
Slow EMT
lt
39
Single Pellet Reaction
External Mass Transfer - 2
(1) The presence of external mass transfer
resistance will only affect the boundary
condition (2) Dimensionless boundary
conditions
x
x
40
Single Pellet Reaction
External Mass Transfer - 3
(3) Biot number (4) Dimensionless equation
41
Single Pellet Reaction
External Mass Transfer - 4
(5) Solving the equation (6)
Concentration profile in spherical pellet
small B means large external mass transfer
resistance large B means no external
mass transfer resistance
42
Single Pellet Reaction
External Mass Transfer - 5
(7) New definition of effectiveness
factor (8) Effectiveness factor versus
Thiele modulus for different Biot numbers
small B means large external mass transfer
resistance large B means no external
mass transfer resistance
43
Single Pellet Reaction
External Mass Transfer - 6
(9) Effects of external mass transfer resistance
slope -1
slope -2
44
Single Pellet Reaction
External Mass Transfer - 7
(10) Summary
45
Single Pellet Reaction
External Mass Transfer - 8
(11) Observed versus intrinsic kinetic parameters
- 1
Reaction-limited
Diffusion-limited
46
Single Pellet Reaction
External Mass Transfer - 9
(11) Observed versus intrinsic kinetic parameters
- 2
Diffusion-limited
Internal mass transfer-limited
External mass transfer-limited
47
Catalyst Pellet
General Balances
(1) Material Balance where
48
Catalyst Pellet
General Balances
(2) Energy Balance where
49
Single Pellet Reaction
Nonisothermal Condition - 1
(1) Material Balance (2) Energy Balance
Practical catalyst pellet usually have high
thermal conductivity and therefore heat transfer
could often be neglected.
50
Single Pellet Reaction
Nonisothermal Condition - 2
(3) Solving the two balance equations for
constant properties therefore
51
Single Pellet Reaction
Nonisothermal Condition - 3
(4) Simplification defining the
dimensionless variables gives
52
Single Pellet Reaction
Nonisothermal Condition - 4
(5) Dimensionless material balance for
nonisothermal pellet Weisz-Hicks
Problem with boundary conditions
53
Single Pellet Reaction
Nonisothermal Condition - 5
(6) Effectiveness factor Weisz-Hicks
Problem (7) Rescaling the Theile
modulus
54
Single Pellet Reaction
Nonisothermal Condition - 6
(8) Effectiveness factor versus Thiele modulus
Weisz-Hicks Problem
Note at large Thiele modulus that asymptotes are
the same for all values of g and b. The
effectiveness factor could be larger than 1 for
some of the parameter values, which becomes more
pronounced for more exothermic reaction. The
interior temperature of the pellet could
be higher than the surface for exothermic
reaction. Multiple steady-state is possible in
the pellet.
55
Single Pellet Reaction
Nonisothermal Condition - 7
(9) Concentration and temperature profiles in
pellet Weisz-Hicks Problem
56
Fixed Bed Reactor
FBR Design 1
Analysis of a fixed bed reactor with a packed bed
of catalyst pellets involves (1) fluid phase
that transports the reactants and products
through the reactor, (2) solid phase where
reaction-diffusion processes occurs.
57
Fixed Bed Reactor
FBR Design 2
(1) Coupling between catalyst and fluid The
two phases communicate by exchanging materials
and energy (2) The following assumptions will be
made for the analysis of a FBR
58
Fixed Bed Reactor
FBR Design 3
(3) Fluid Phase (a) mole balance
(b) energy balance (c) pressure drop
(Ergun Equation)
59
Fixed Bed Reactor
FBR Design 4
(4) Catalyst pellet (a) mole balance
(b) energy balance
60
Fixed Bed Reactor
FBR Design 5
(5) Coupling between fluid and catalyst phases
(a) mole balance (b) energy
balance
61
Fixed Bed Reactor
FBR Design 6
(6) Quick summary
62
Fixed Bed Reactor
FBR Design 7
(7) Simple examples
The first order, irreversible reaction took place
in a 0.3 cm radius spherical catalyst pellet at T
450 K. The feed to the reactor is pure A (12
mol/s, 1.5 atm), the pellets production rate is
2.5 x 10-5 mol/g-s. The bed density is given to
be 0.6 g/cm3. Assume that the reactor operates
isothermally at 450 K. External mass-transfer
limitations are negligible. Given
Find the FBR volume needed for 97 conversion of
A.
63
Fixed Bed Reactor
FBR Design 8
(7a) FBR design equation (7b) First order,
irreversible reaction Thiele modulus is
independent of concentration (7c)
Effectiveness factor is constant along the axial
length
64
Fixed Bed Reactor
FBR Design 9
(7d) Concentration in term of molar
flow (7e) Substituting into the FBR design
equation
65
Fixed Bed Reactor
FBR Design 9
(7f) What happen when there is external diffusion
resistance let